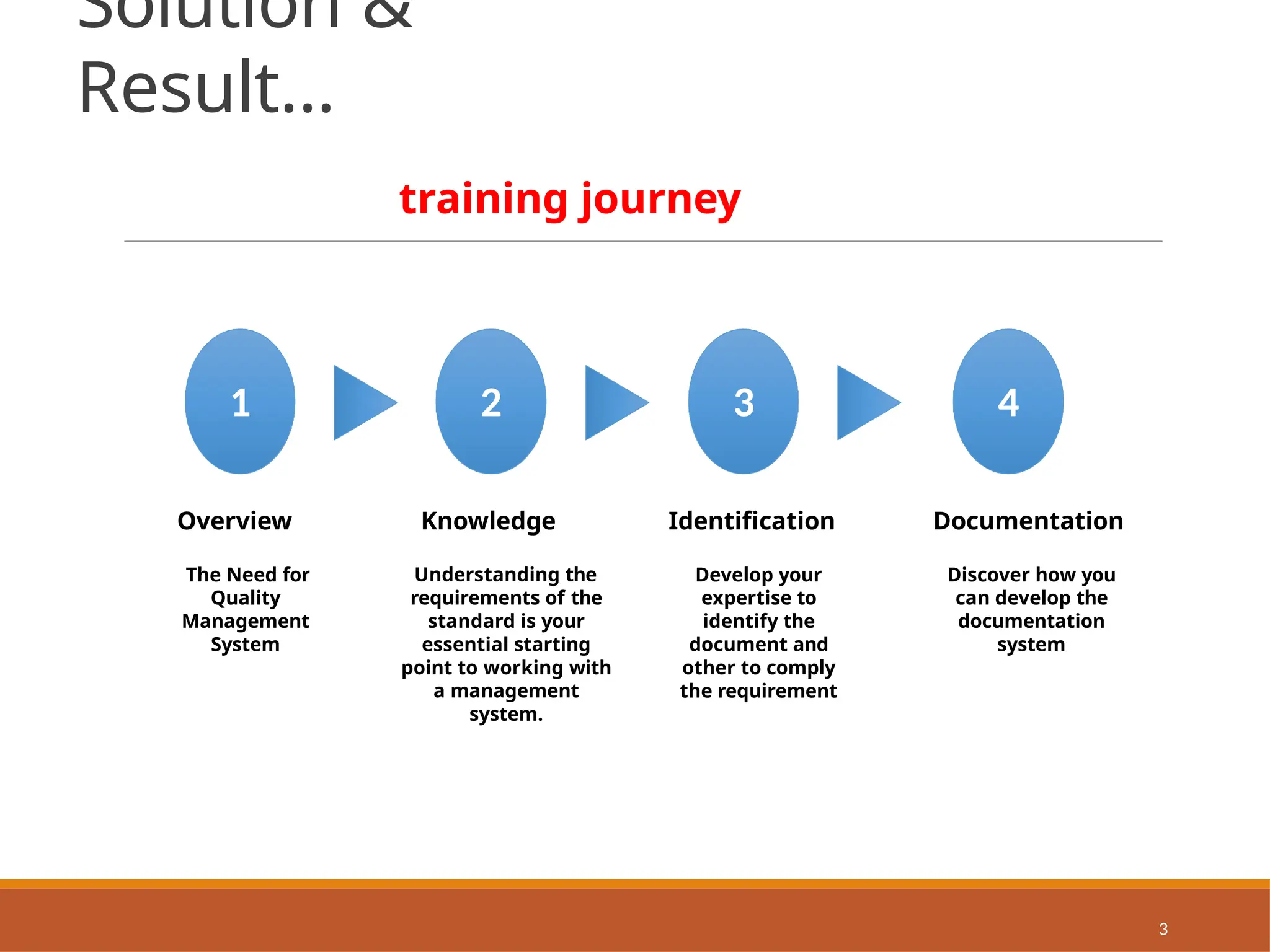
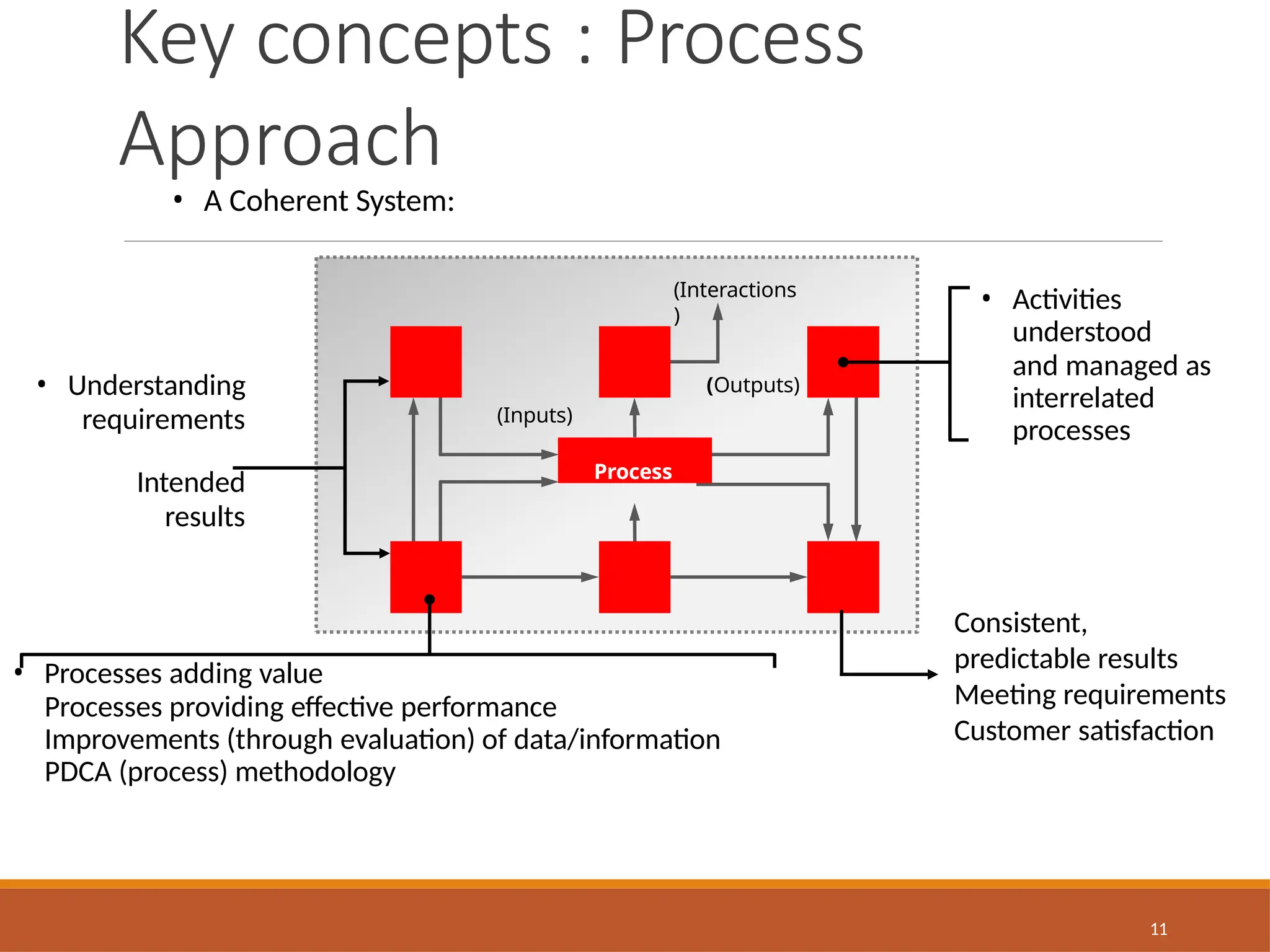
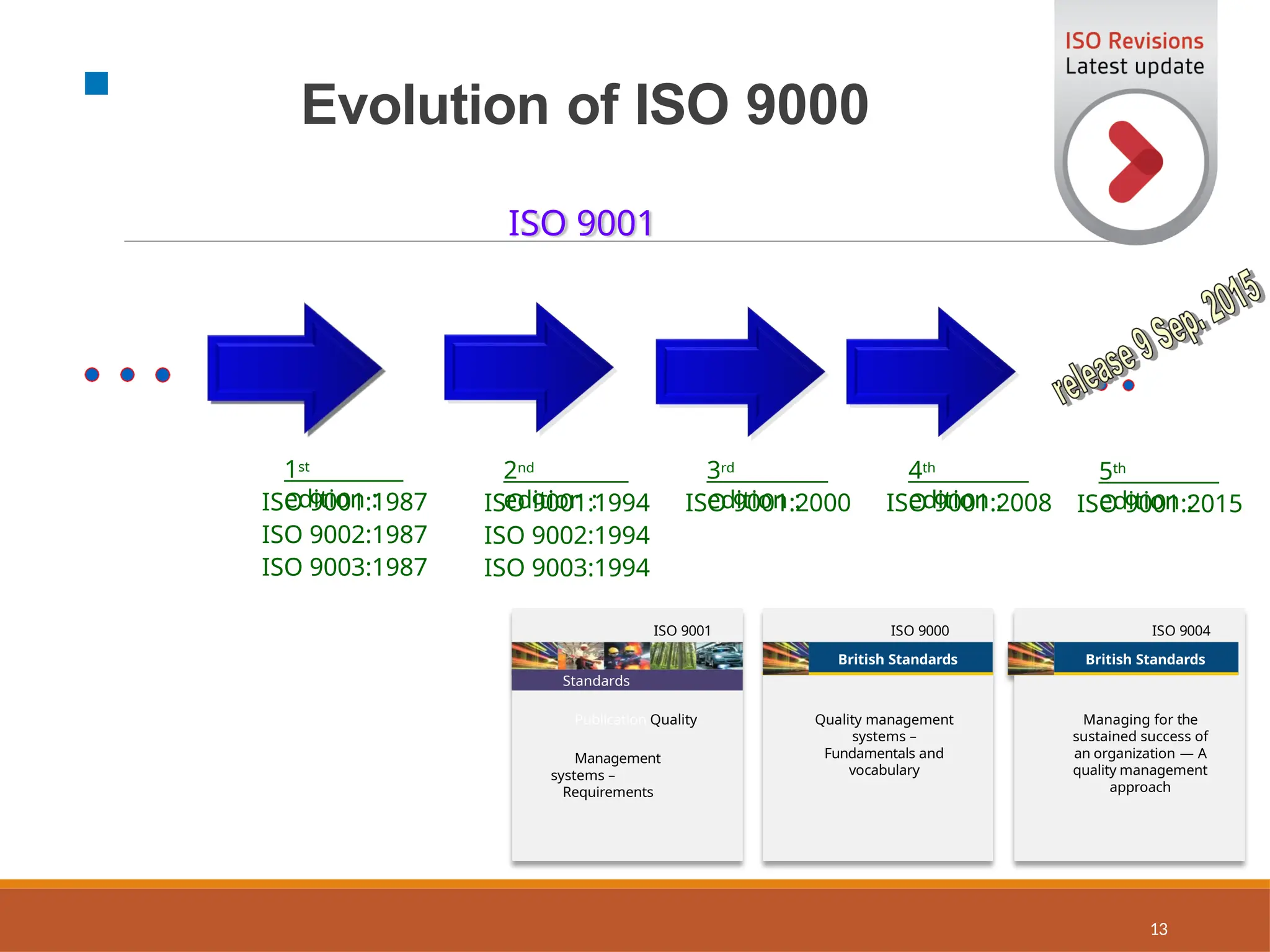
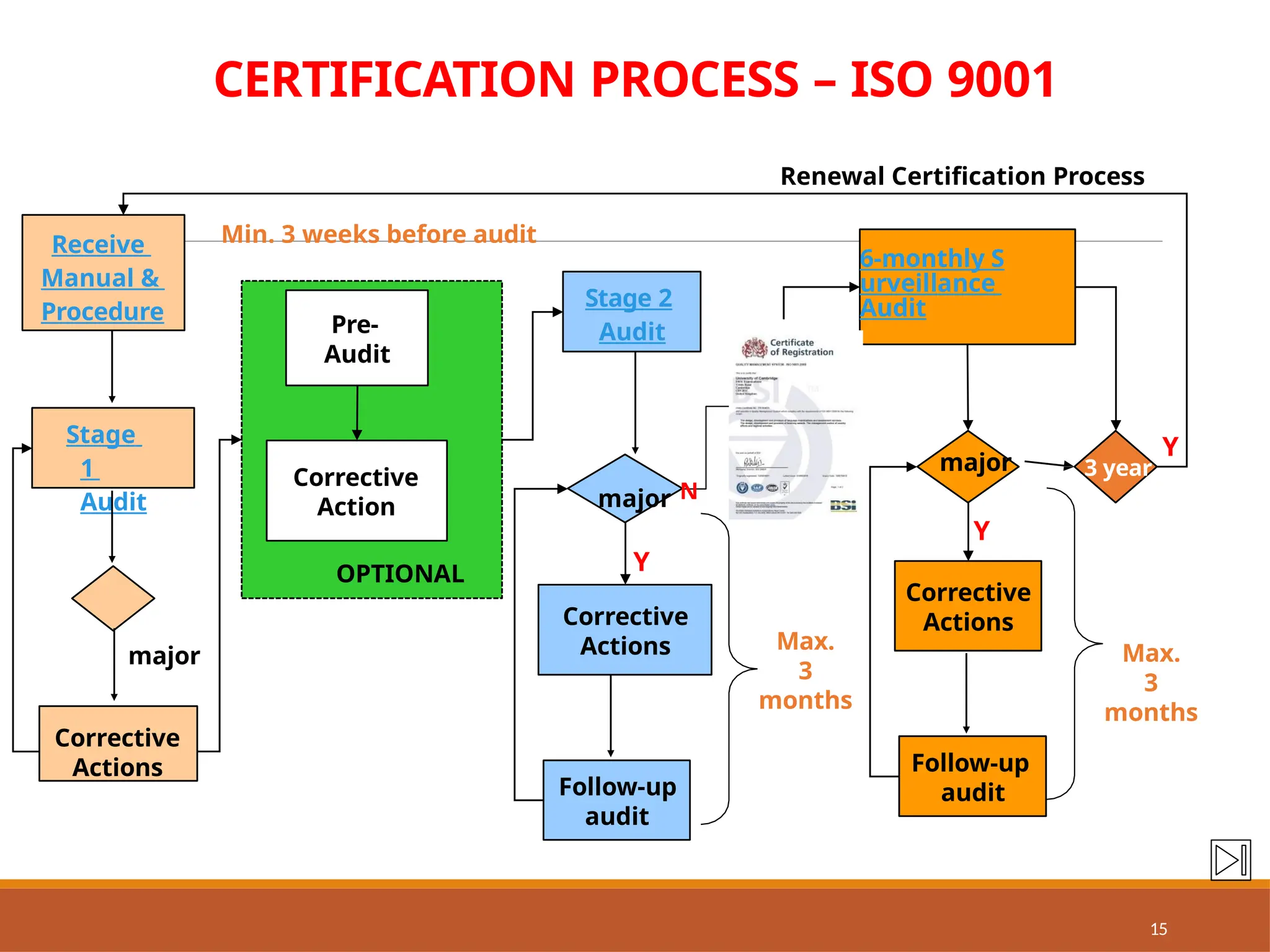
The document provides an extensive overview of ISO 9001:2015 Quality Management Systems, detailing the requirements for establishing a QMS, its benefits, key concepts, and principles. It outlines the process for developing documentation, conducting risk and opportunity assessments, and understanding the organization’s context in relation to stakeholder expectations. Additionally, it emphasizes the importance of continual improvement and stakeholder satisfaction in managing quality standards.