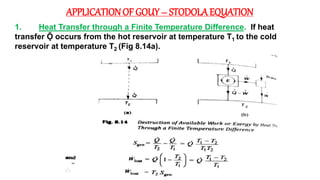
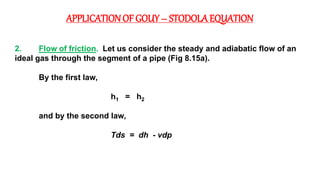
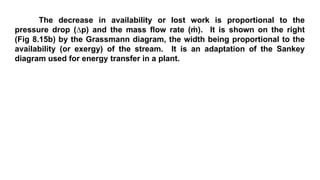
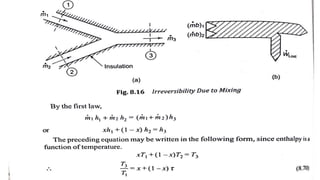
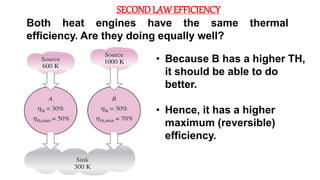
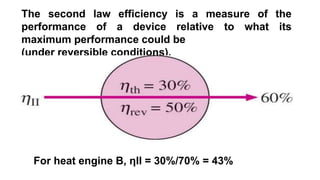
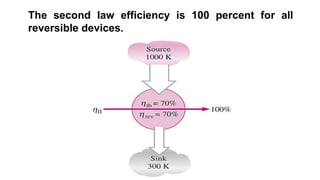
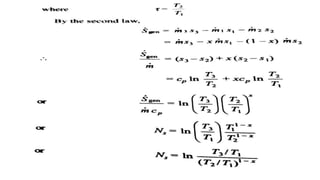The document discusses irreversibility, the Gouy-Stodola theorem, and its applications to calculating second law efficiency. It states that the Gouy-Stodola theorem establishes that the rate of exergy (available energy) loss in a process is proportional to the rate of entropy generation. It then provides examples of applying the Gouy-Stodola equation to heat transfer through a finite temperature difference, fluid flow with friction, and mixing of two fluids. Finally, it defines second law efficiency as a measure of a device's performance relative to its maximum possible reversible performance.