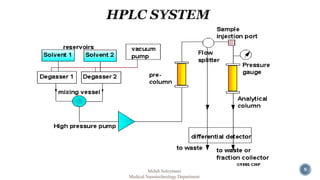
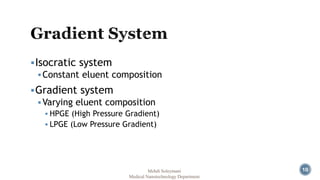
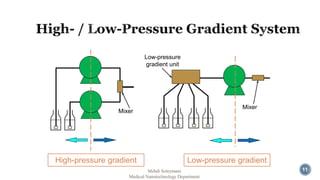
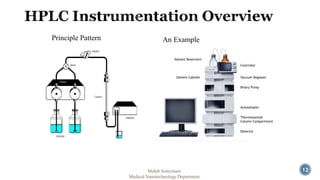
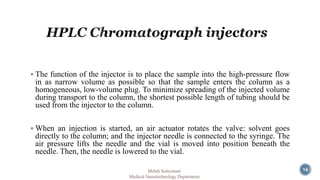
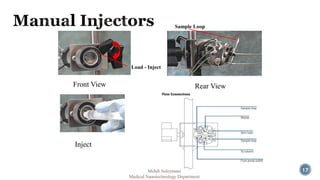
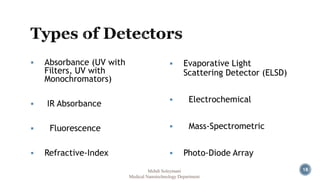
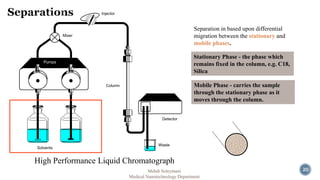
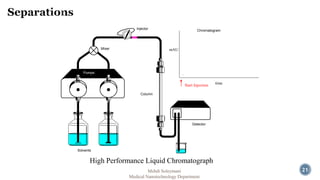
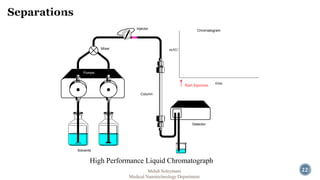
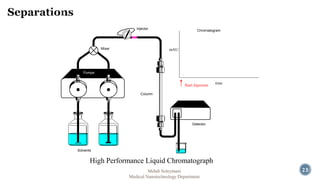
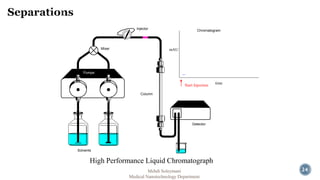
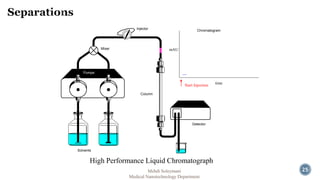
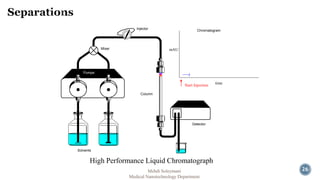
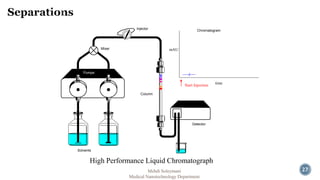
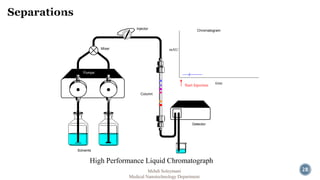
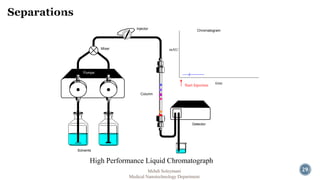
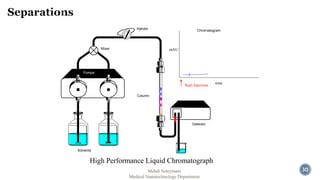
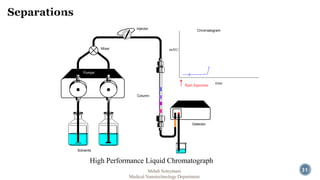
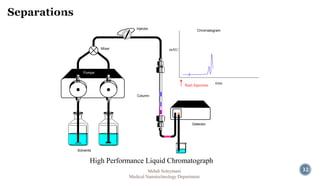
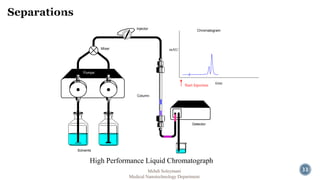
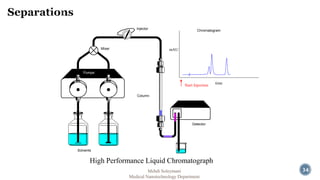
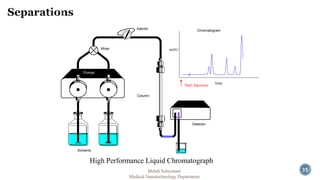
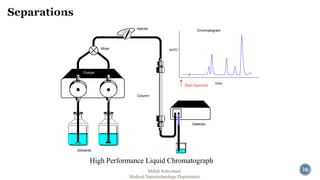
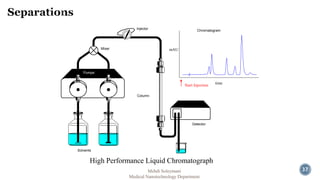
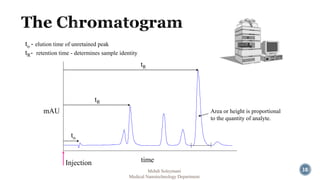
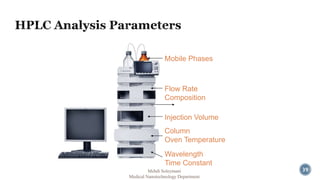
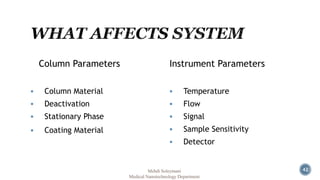
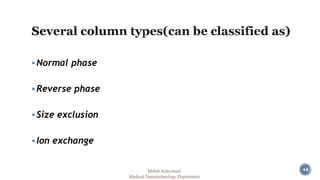
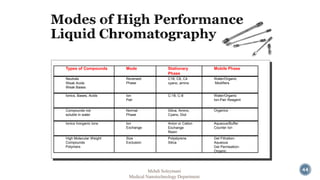
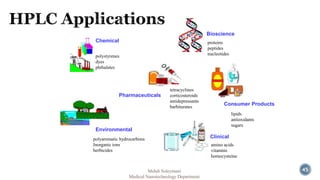
The document presents an overview of high performance liquid chromatography (HPLC). It discusses the key components of an HPLC system including the reservoir, pump, injector, separation column, and detector. It explains that compounds are separated on the column based on differences in how they partition between the mobile and stationary phases. The document also reviews different modes of HPLC, common applications, and advantages over gas chromatography.