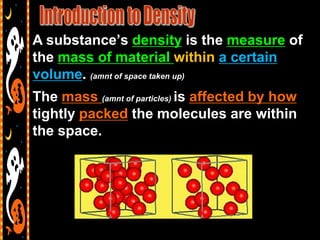
This document provides instructions and materials for a lesson on density. It includes:
1) A list of materials needed for the lesson, including pencils, paper, and a density introduction page.
2) Practice problems on density, volume, and states of matter.
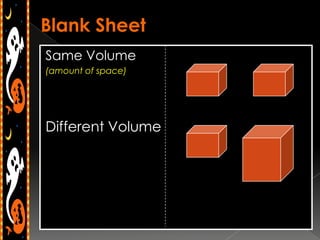
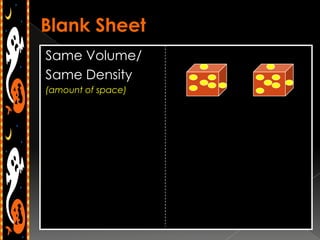
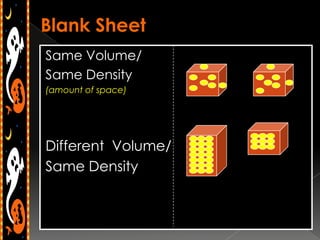
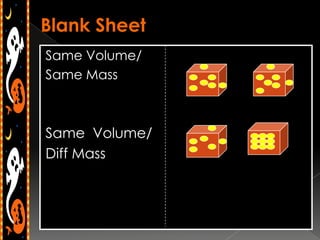
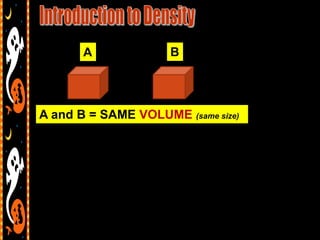
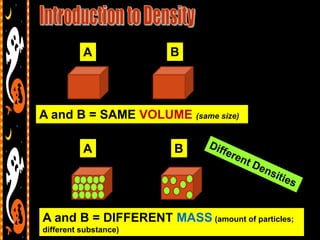
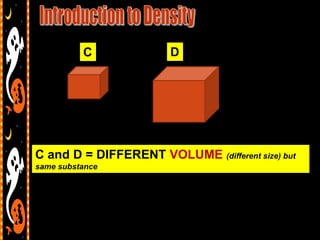
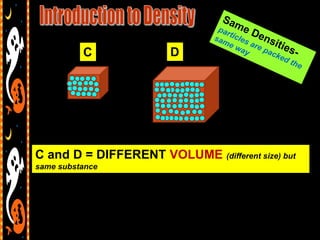
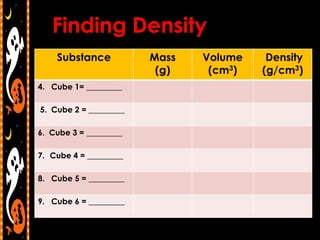
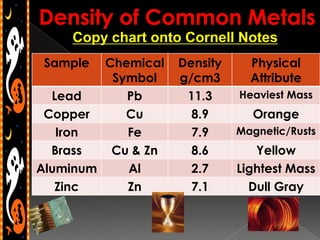
3) Instructions for an identifying volume lab and examples comparing objects with the same and different volumes, masses, and densities to understand these concepts.