The document provides a review of key concepts related to volume, density, and mass including:
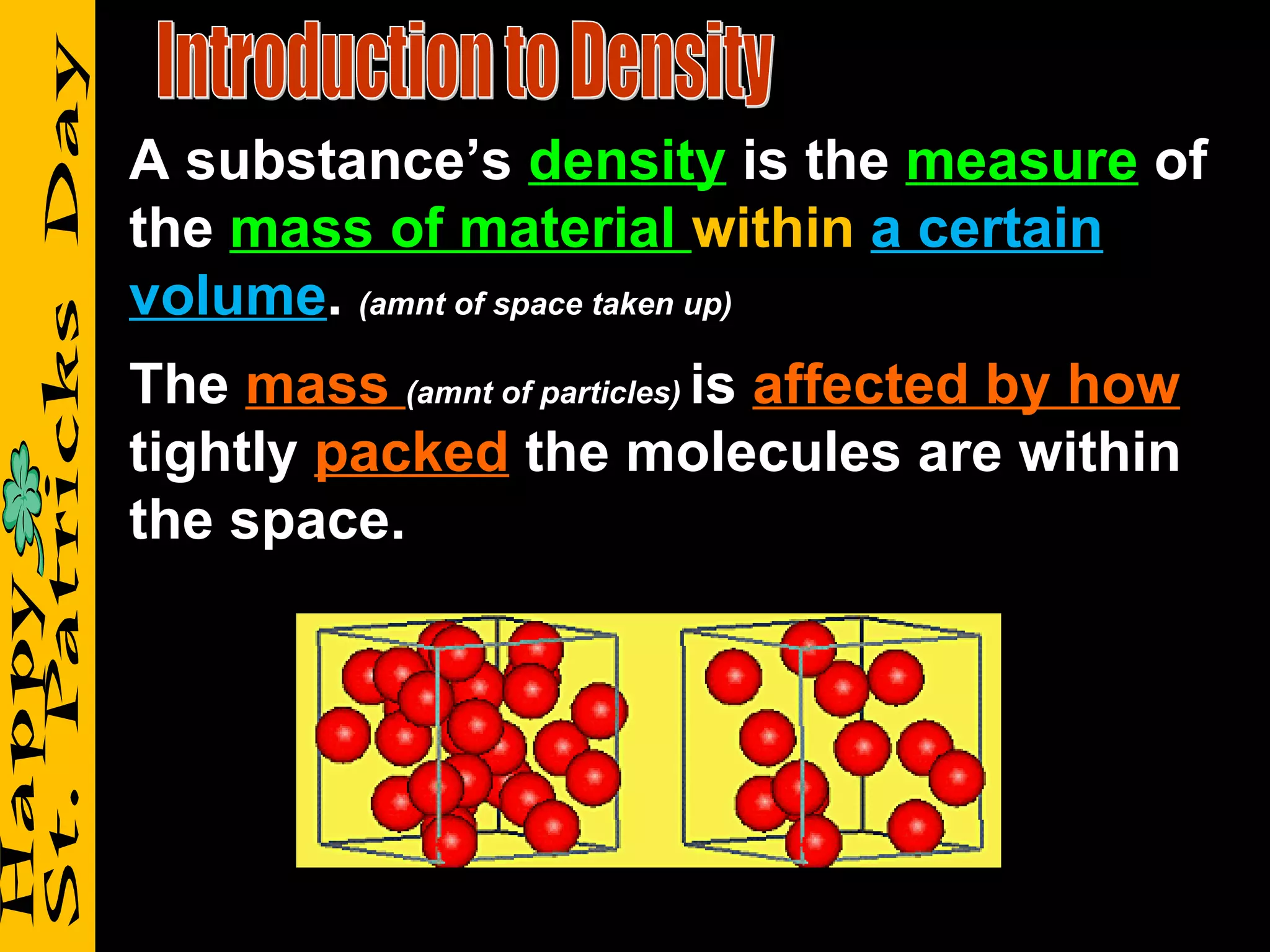
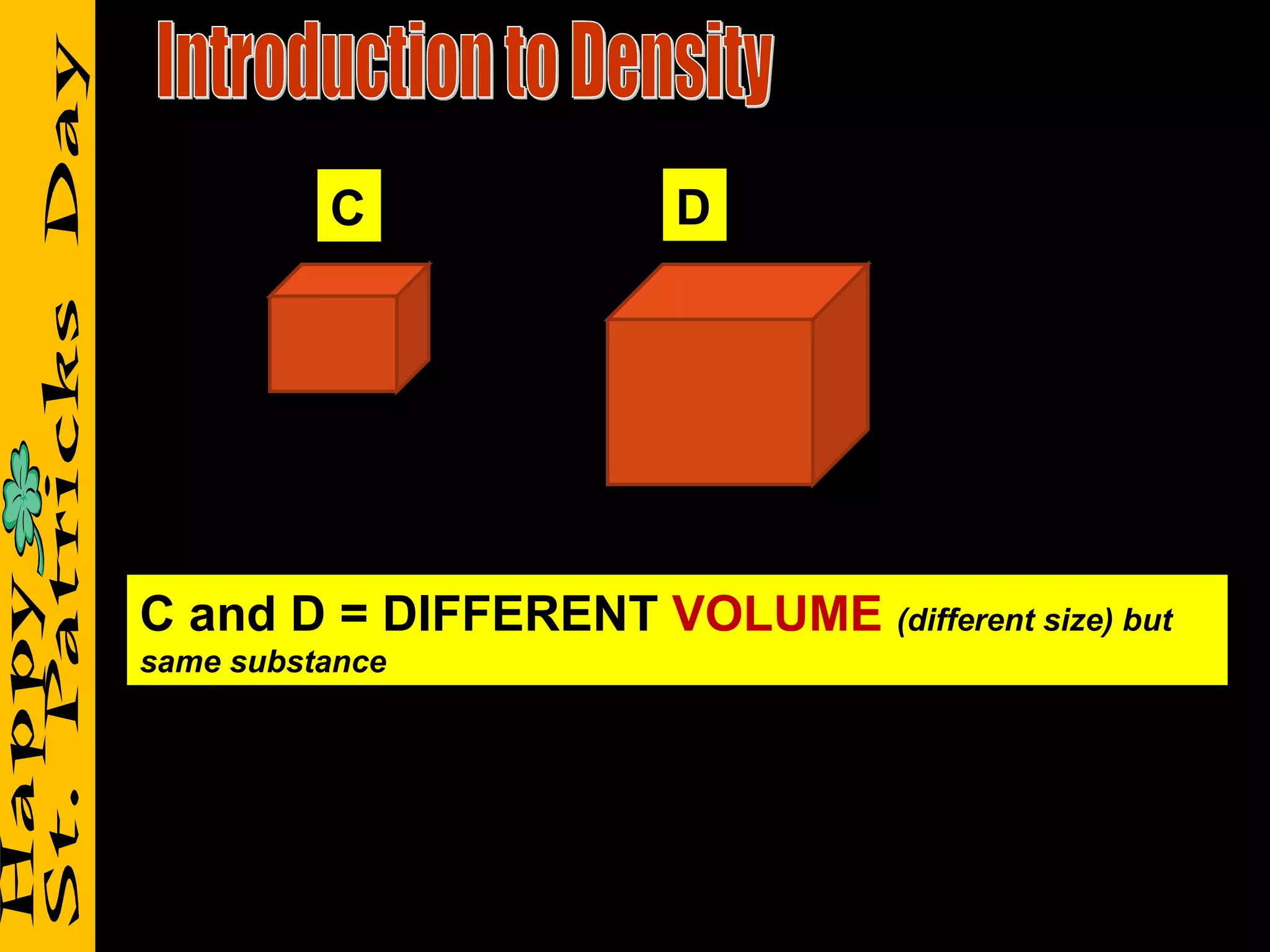
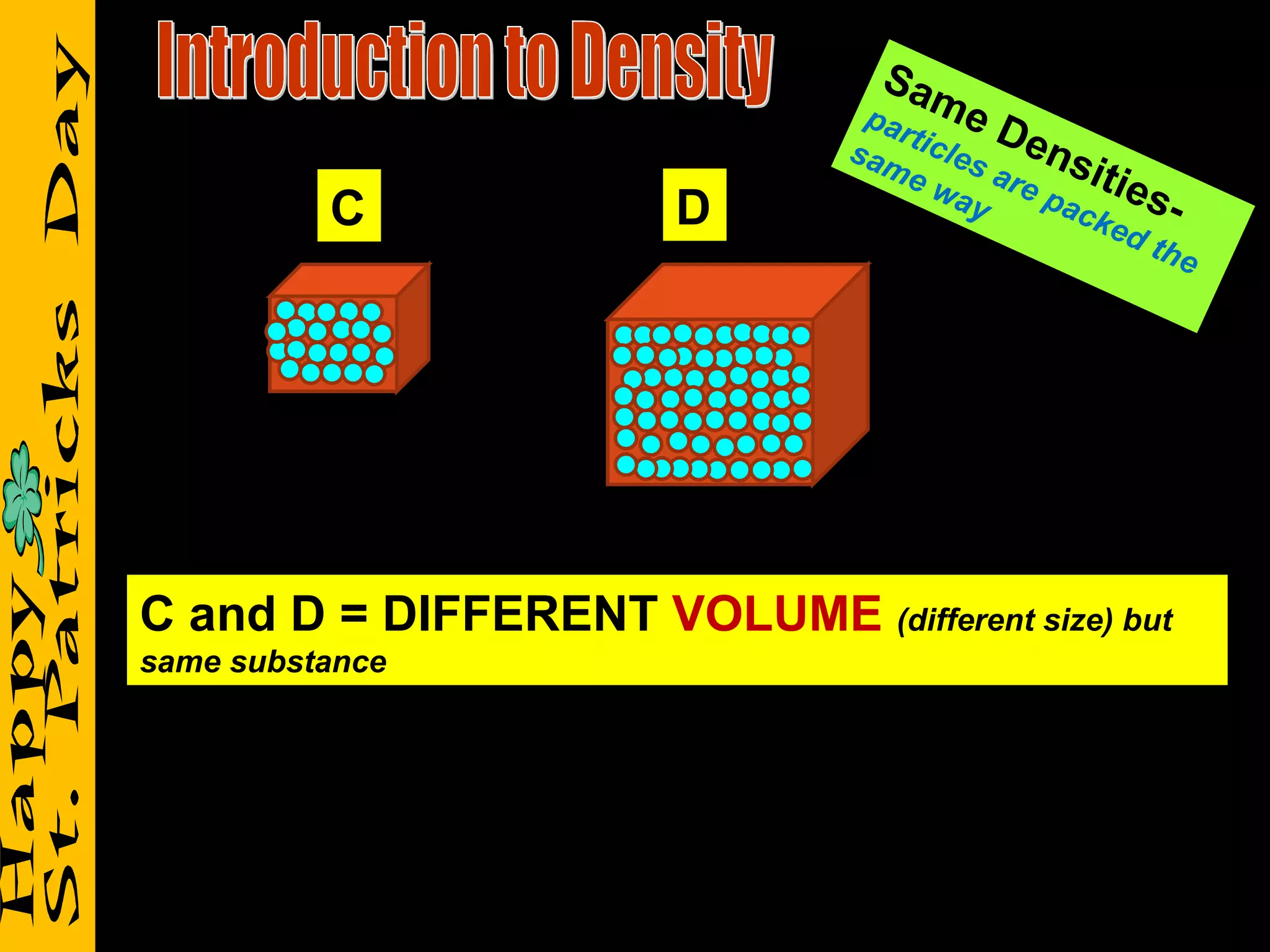
1) It defines density as a measure of the mass of a substance within a certain volume, and explains how tightly packed molecules affect density.
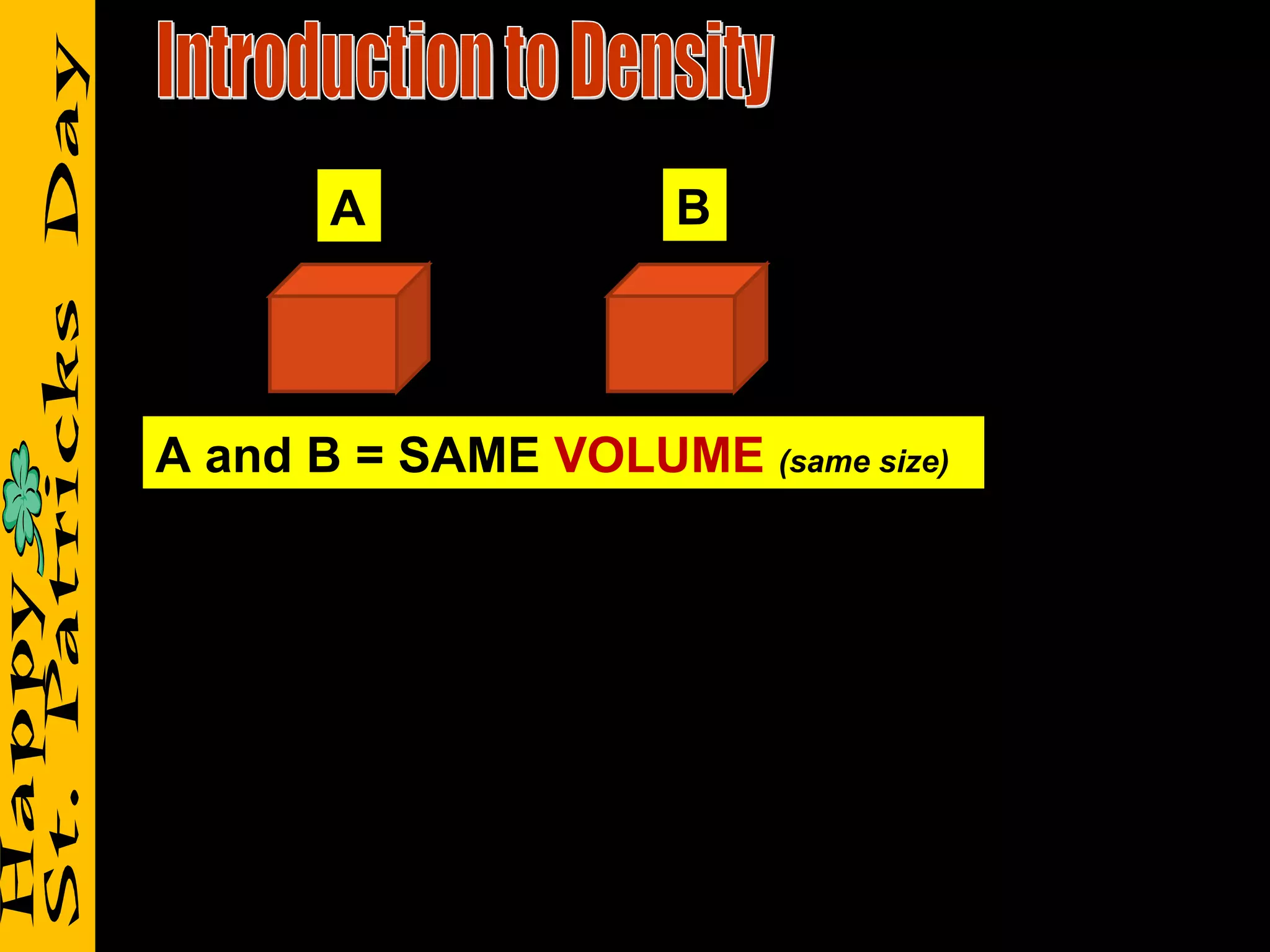
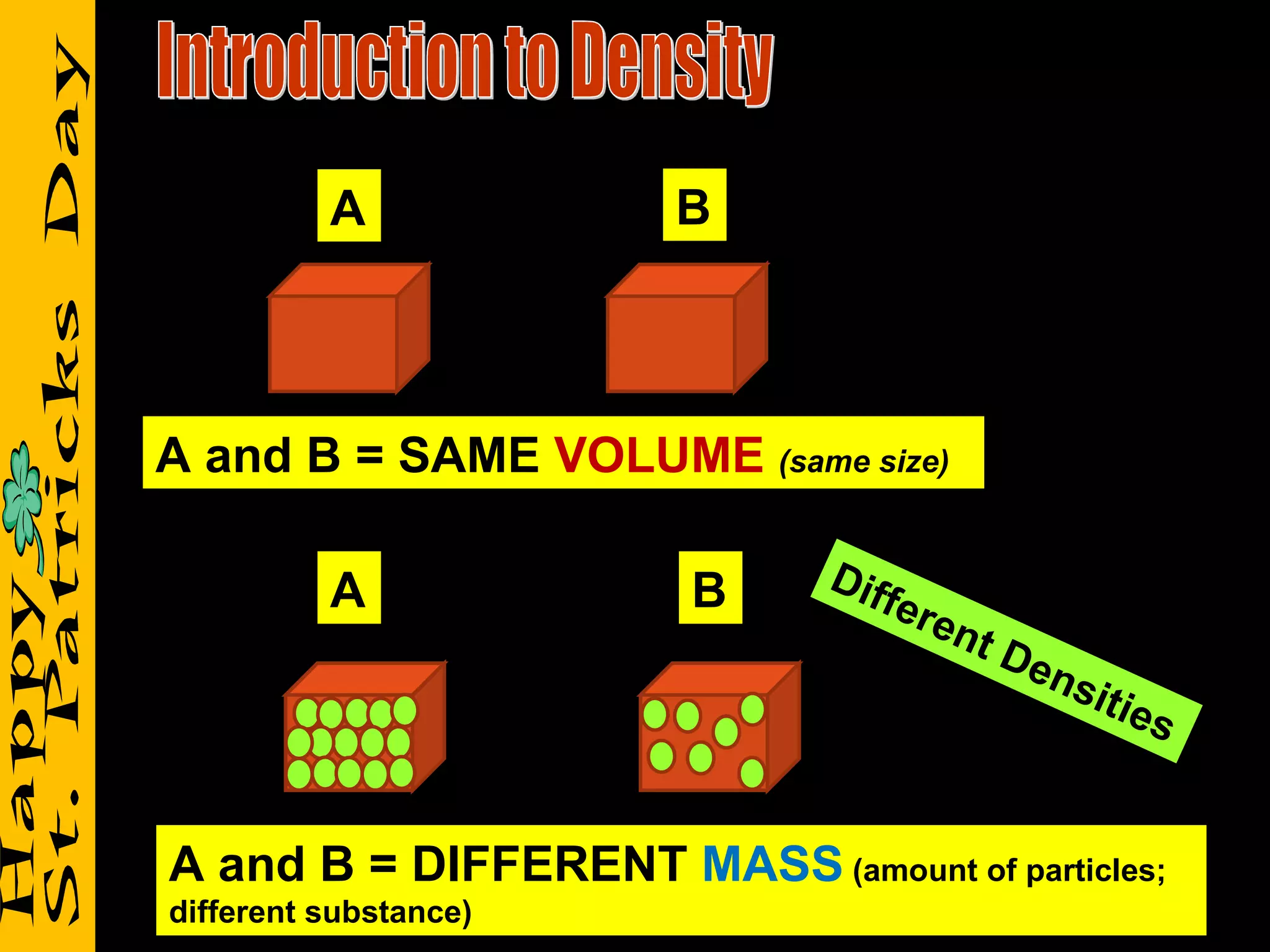
2) It reviews vocabulary terms like volume, density, and mass and provides examples of how these concepts relate for objects with the same or different volumes/masses.
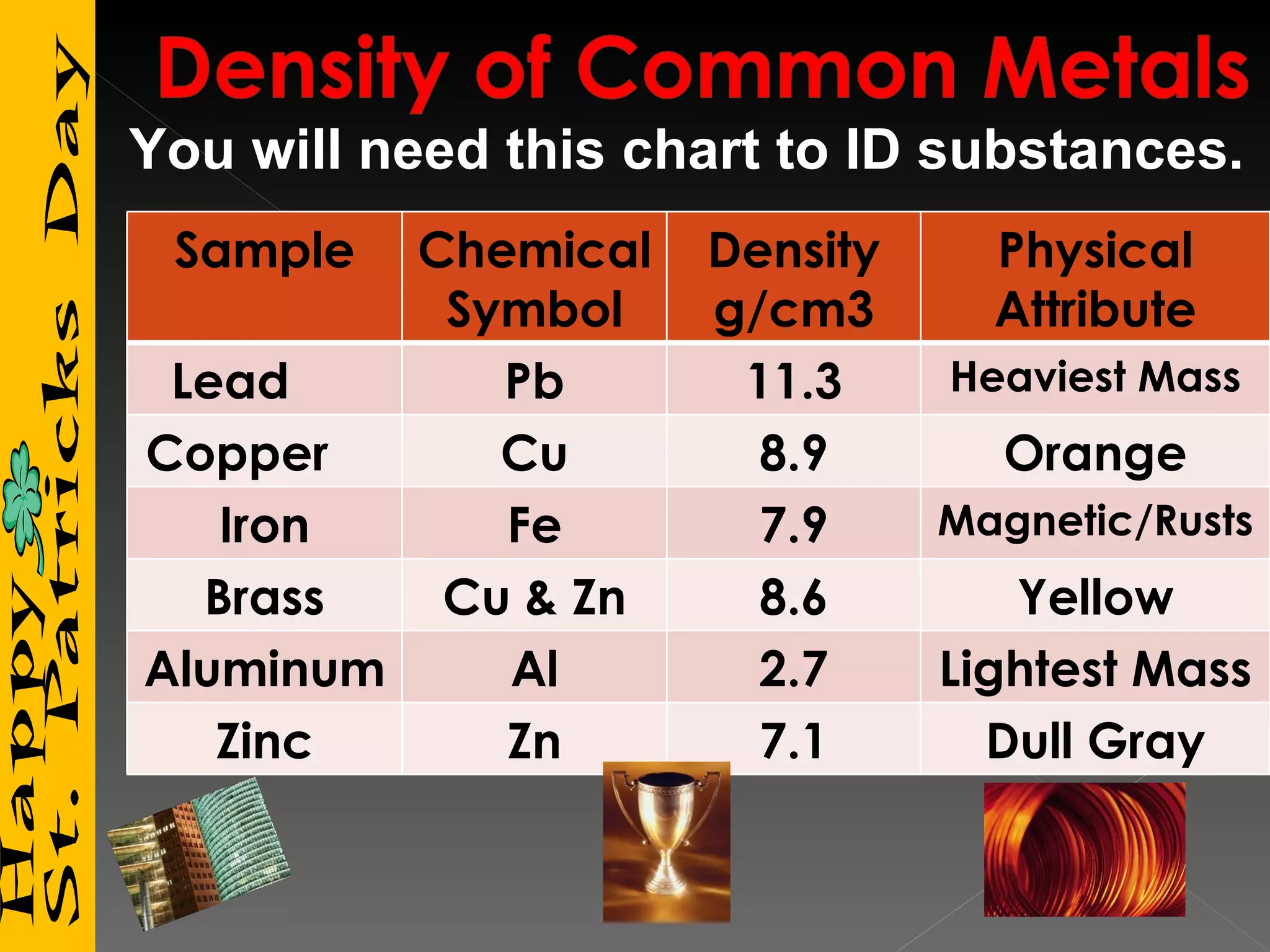
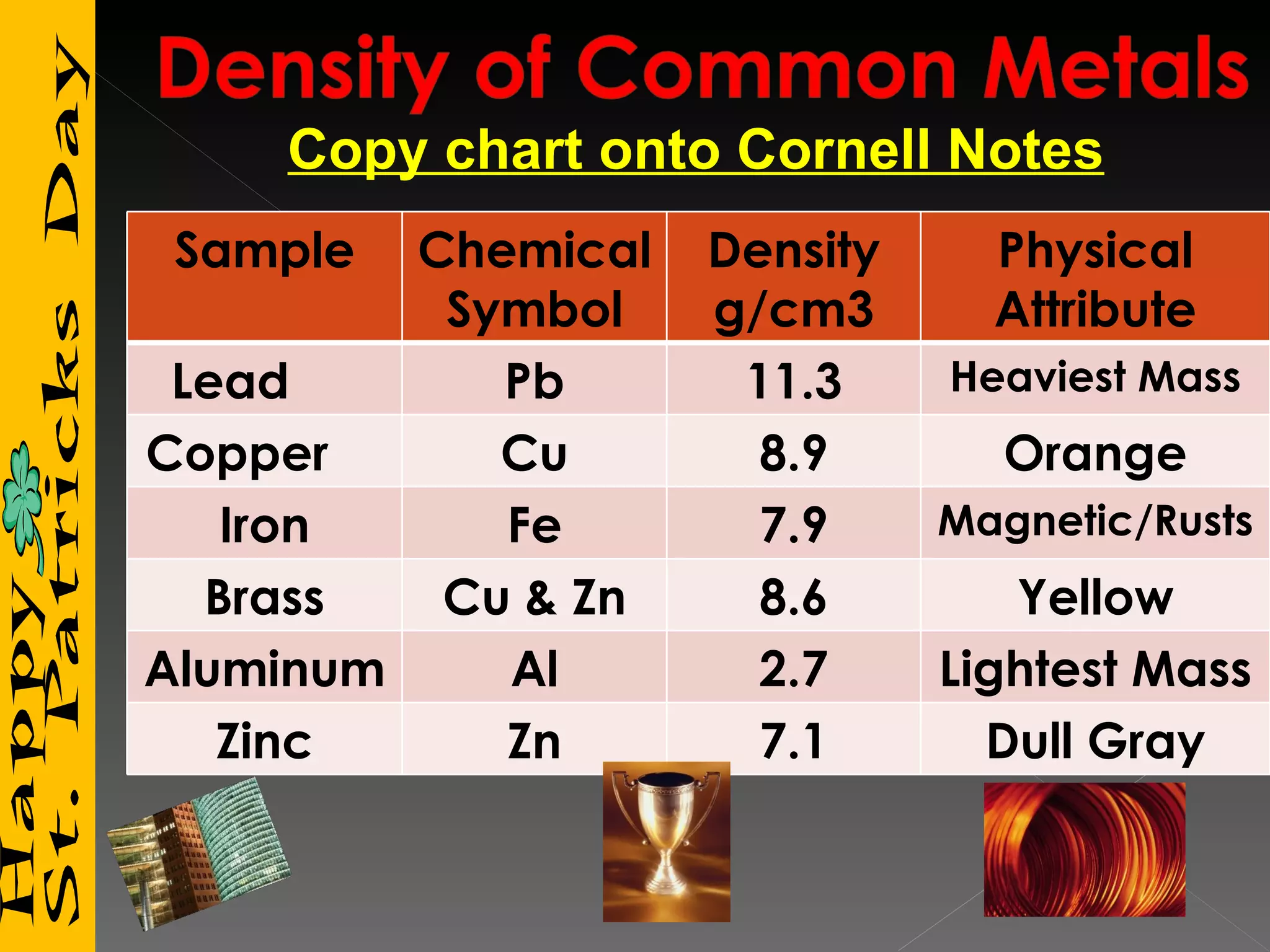
3) It includes a chart listing common substances with their densities to help identify unknown materials.