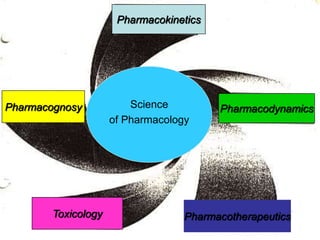
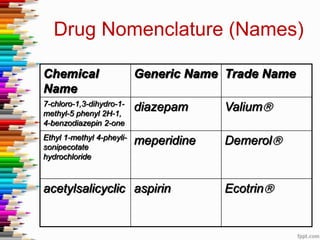
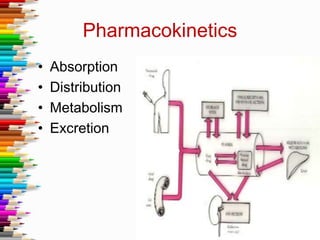
This document defines key terms and concepts in pharmacology. It discusses how pharmacology is the study of drug interactions with living systems. The four main processes that determine a drug's effects are absorption, distribution, metabolism, and excretion. Factors like genetics, age, and health conditions can influence how individuals respond to drugs. Adverse reactions, drug interactions, and tolerance are important considerations when using medications. Standards and regulations aim to ensure drugs are safe, effective, and produced consistently.