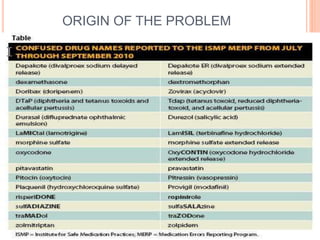
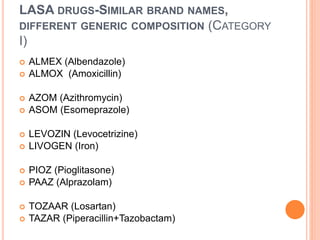
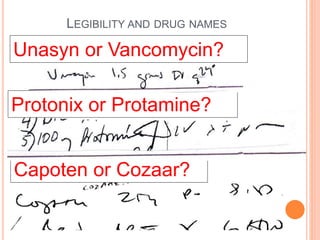
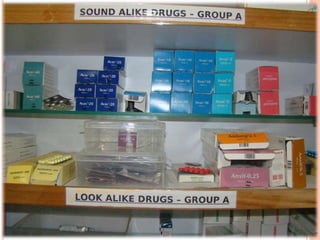
The document discusses the prevalence and dangers of look-alike and sound-alike (LASA) drug names, which often lead to medication errors that can cause patient harm or death. It classifies various categories of LASA drugs, outlines the major sources of errors, and proposes several solutions to mitigate these risks, such as improving prescriber handwriting and implementing tall man lettering. The document emphasizes the importance of awareness among healthcare professionals and patients to prevent medication errors.