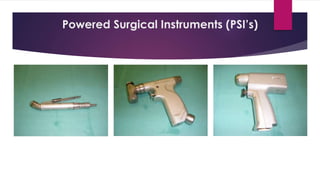
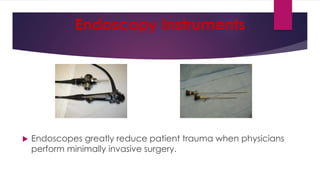
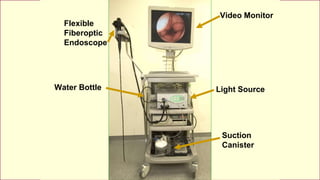
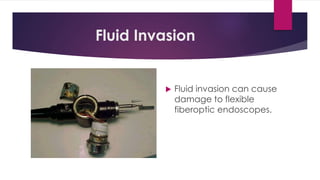
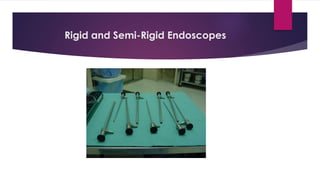
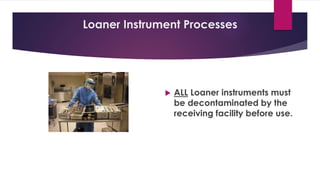
This document discusses complex surgical instruments used in medical procedures. It provides an overview of powered surgical instruments, which can be electrically, pneumatically, or battery powered. These instruments are delicate and difficult to clean due to their complex internal mechanisms. The document outlines the multi-step process required to properly clean, disinfect, and sterilize powered instruments according to manufacturer guidelines. It also discusses endoscopic instruments, including flexible fiberoptic and rigid endoscopes. Endoscopes pose challenges for cleaning due to internal channels and crevices. The document stresses the importance of following regulations and guidelines as well as manufacturer instructions to safely reprocess these specialized medical devices.