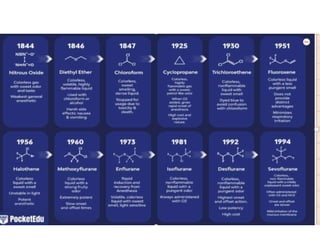
1. Several gases were discovered to have anesthetic properties throughout the 19th century, including nitrous oxide, diethyl ether, and chloroform.
2. Between 1920-1940, newer inhaled anesthetics like ethylene, cyclopropane, and divinyl ether were introduced with faster induction and recovery times but also serious drawbacks like flammability and toxicity.
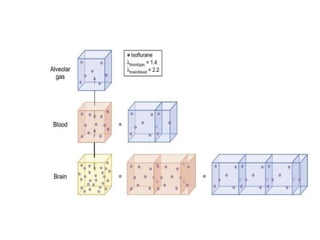
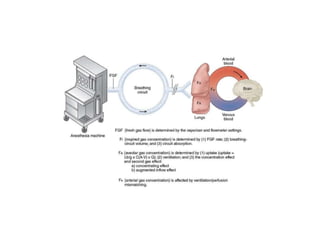
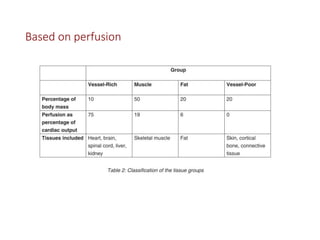
3. Modern inhaled anesthetics developed through fluorination have greater stability and lesser toxicity compared to earlier agents. Their uptake and distribution involves transfer between the anesthesia machine, breathing circuit, alveoli, and pulmonary blood based on partial pressures and solubility properties.