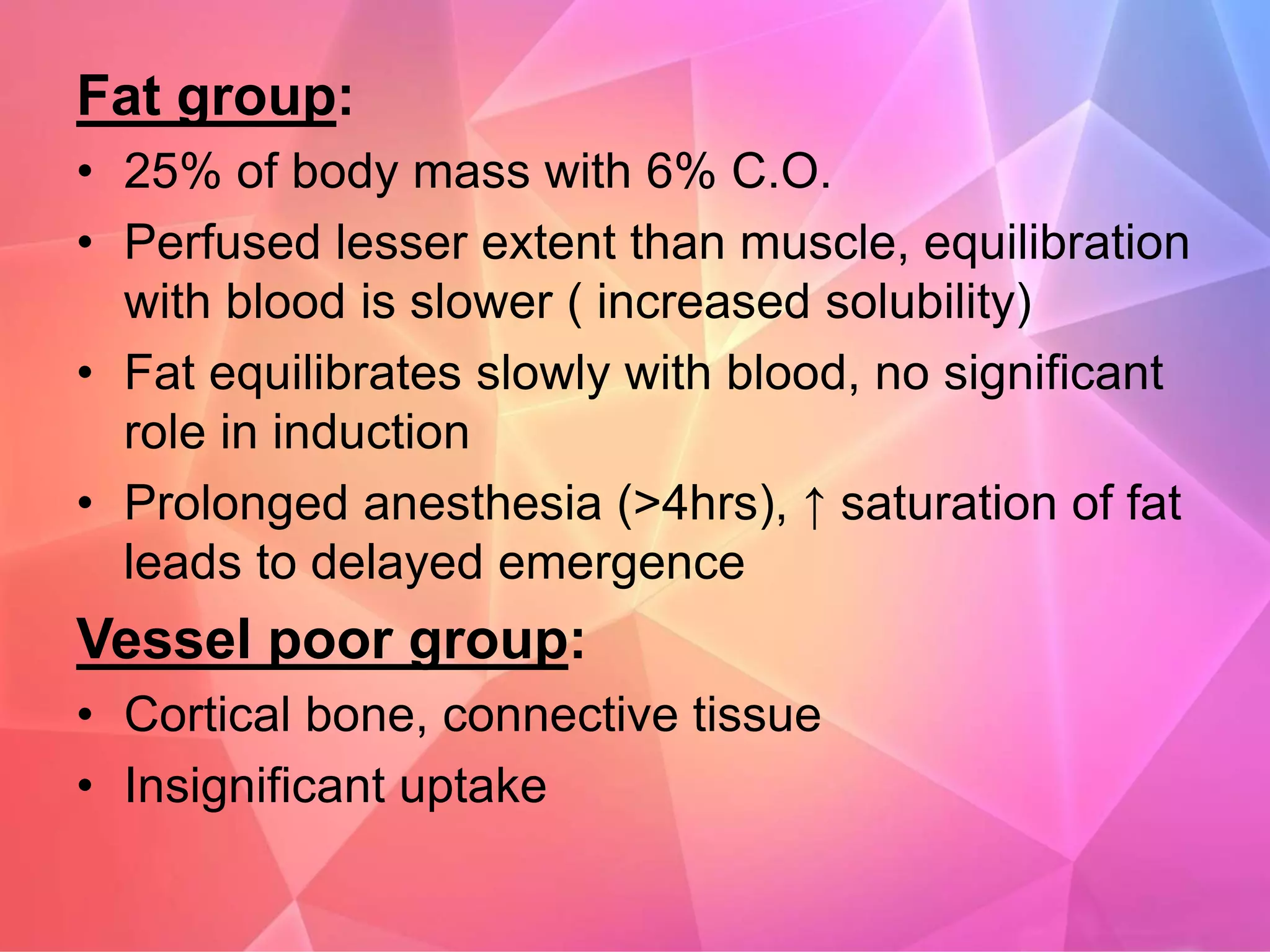
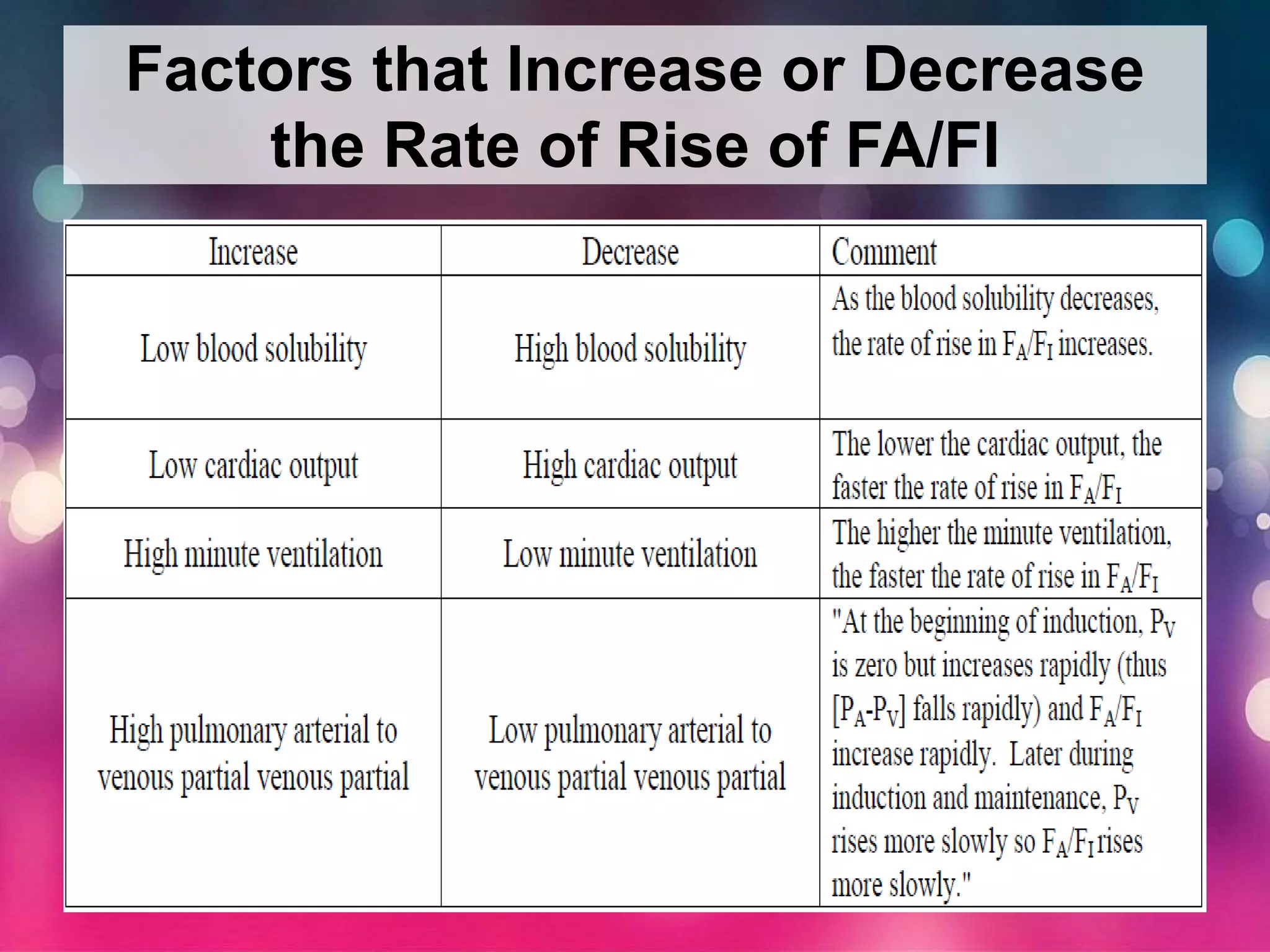
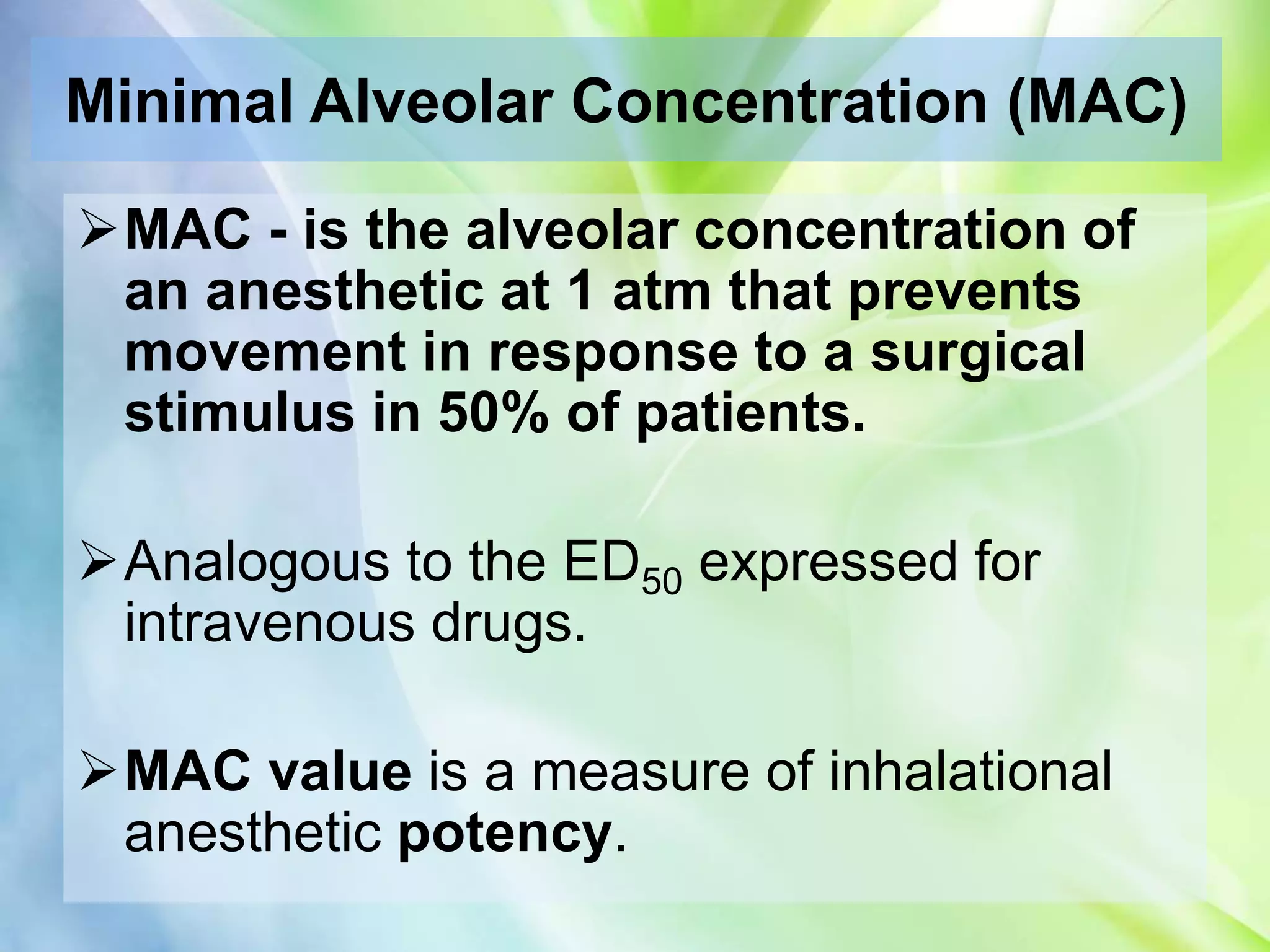
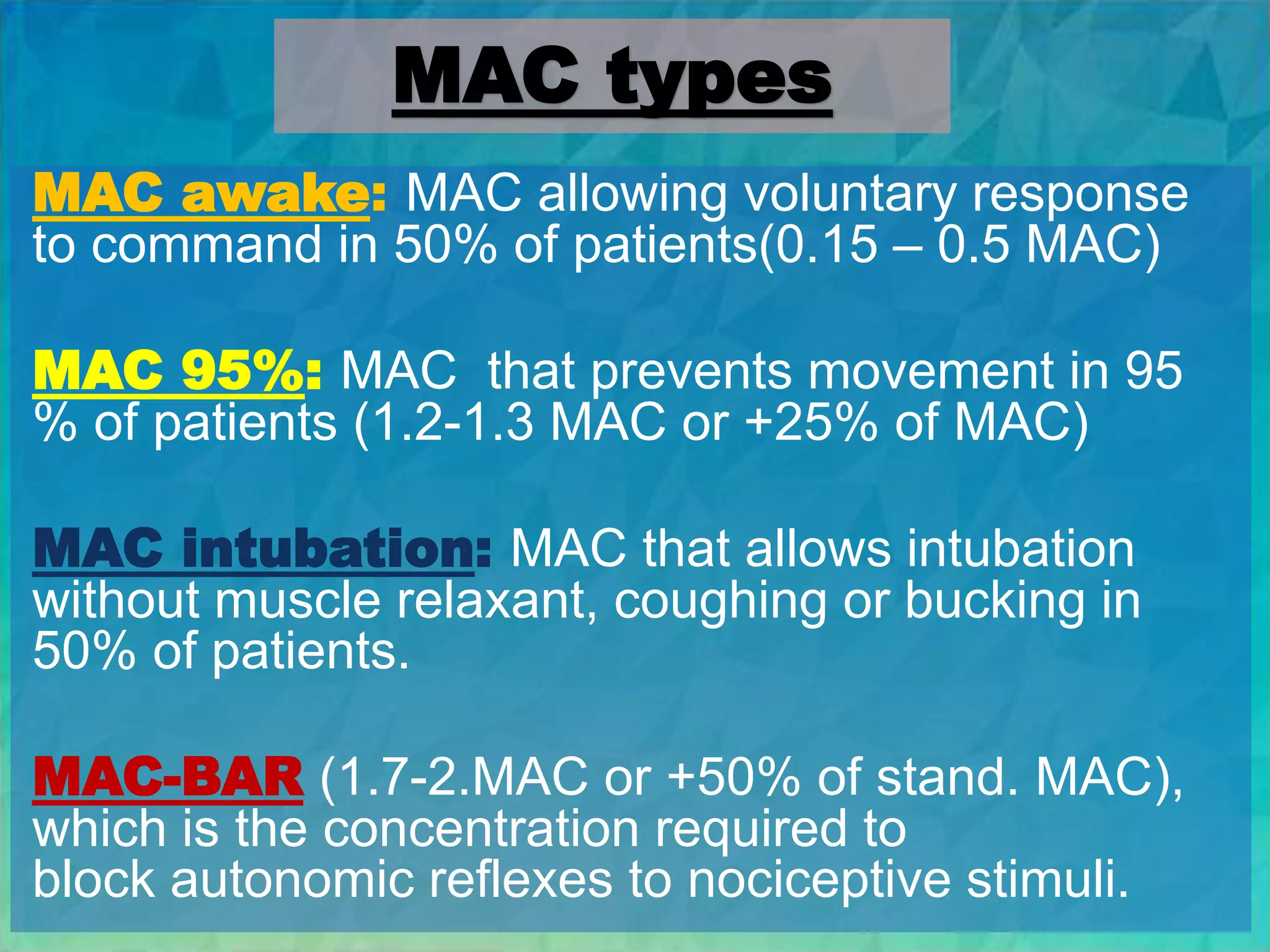
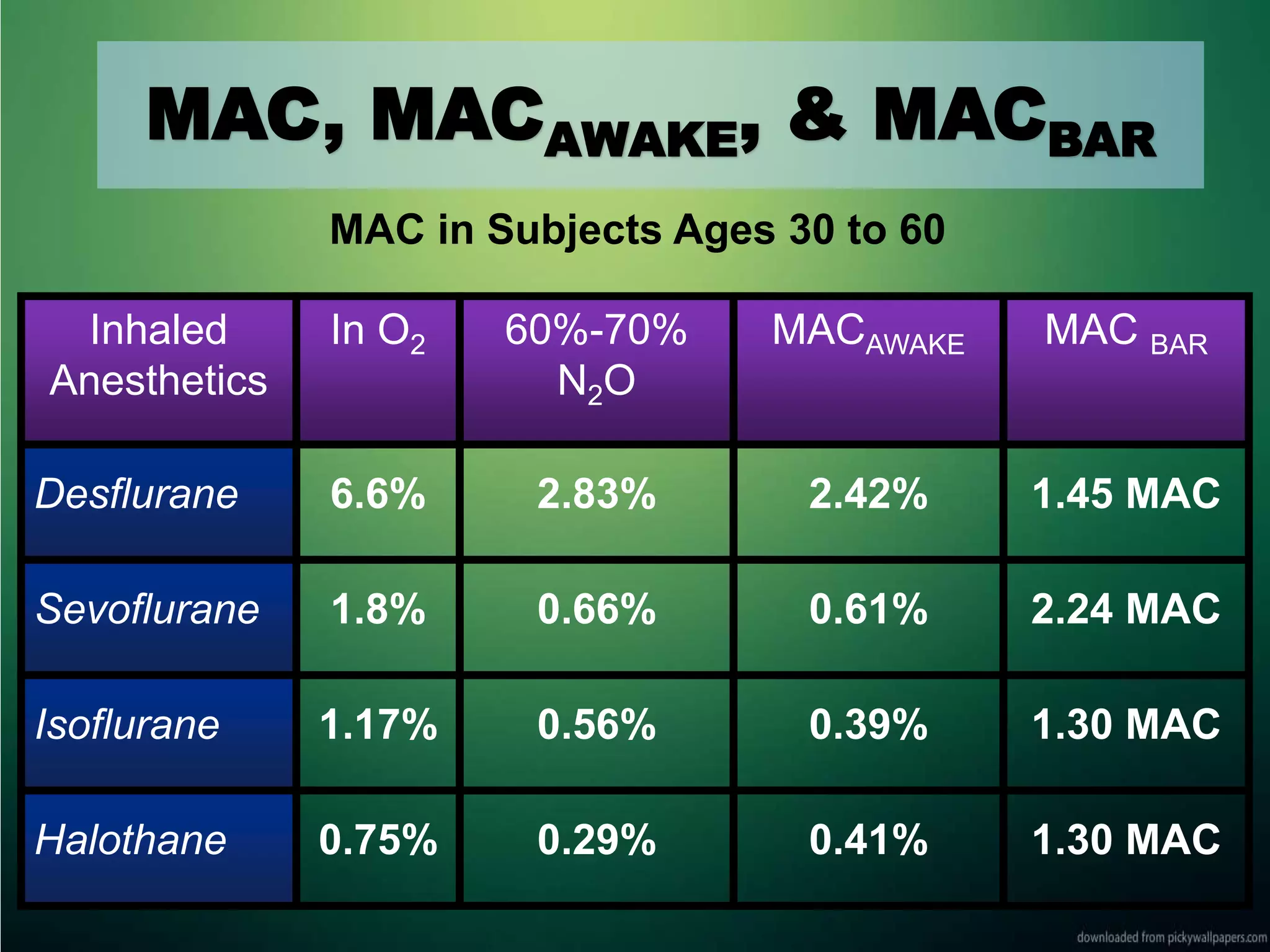
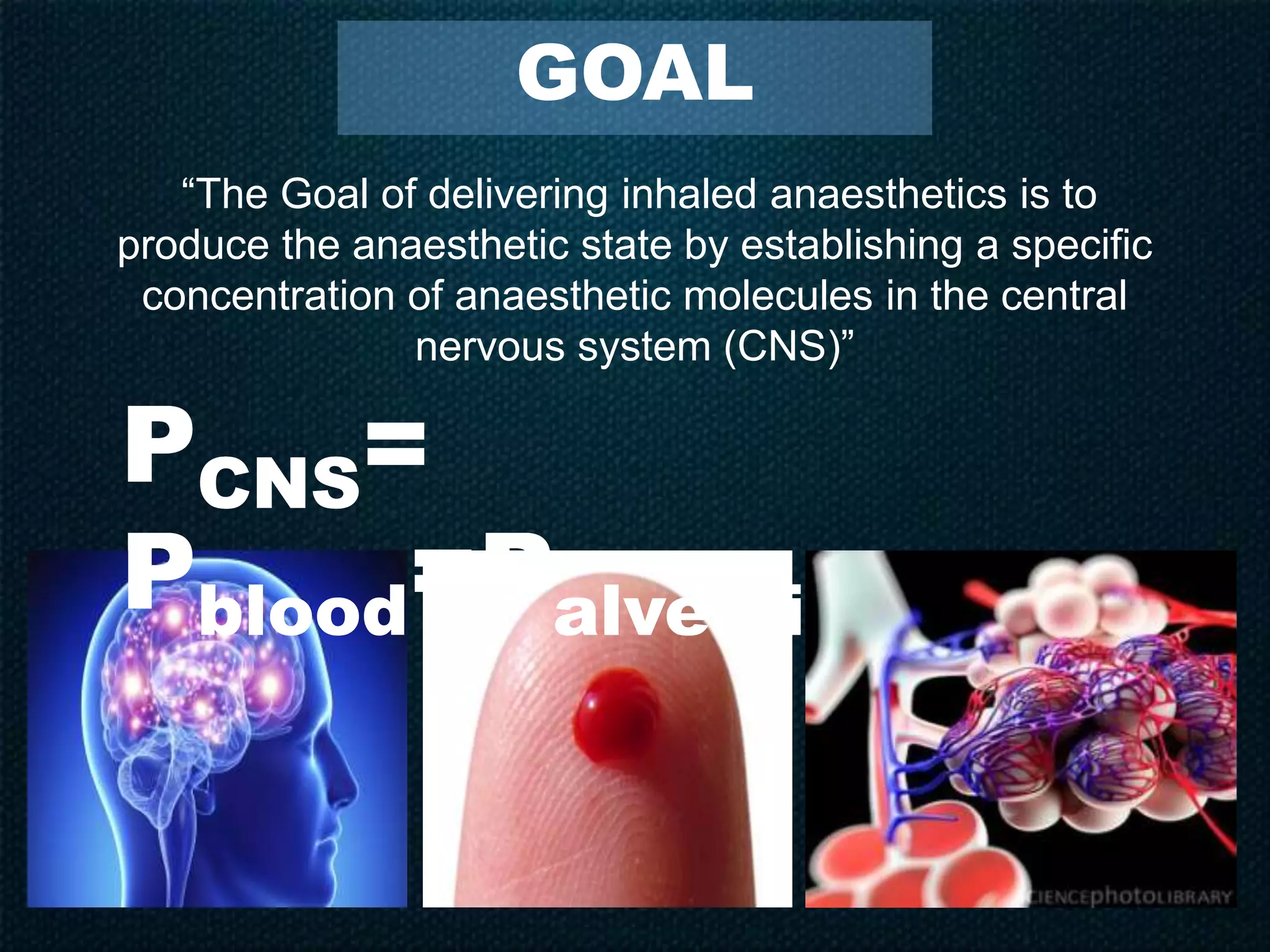
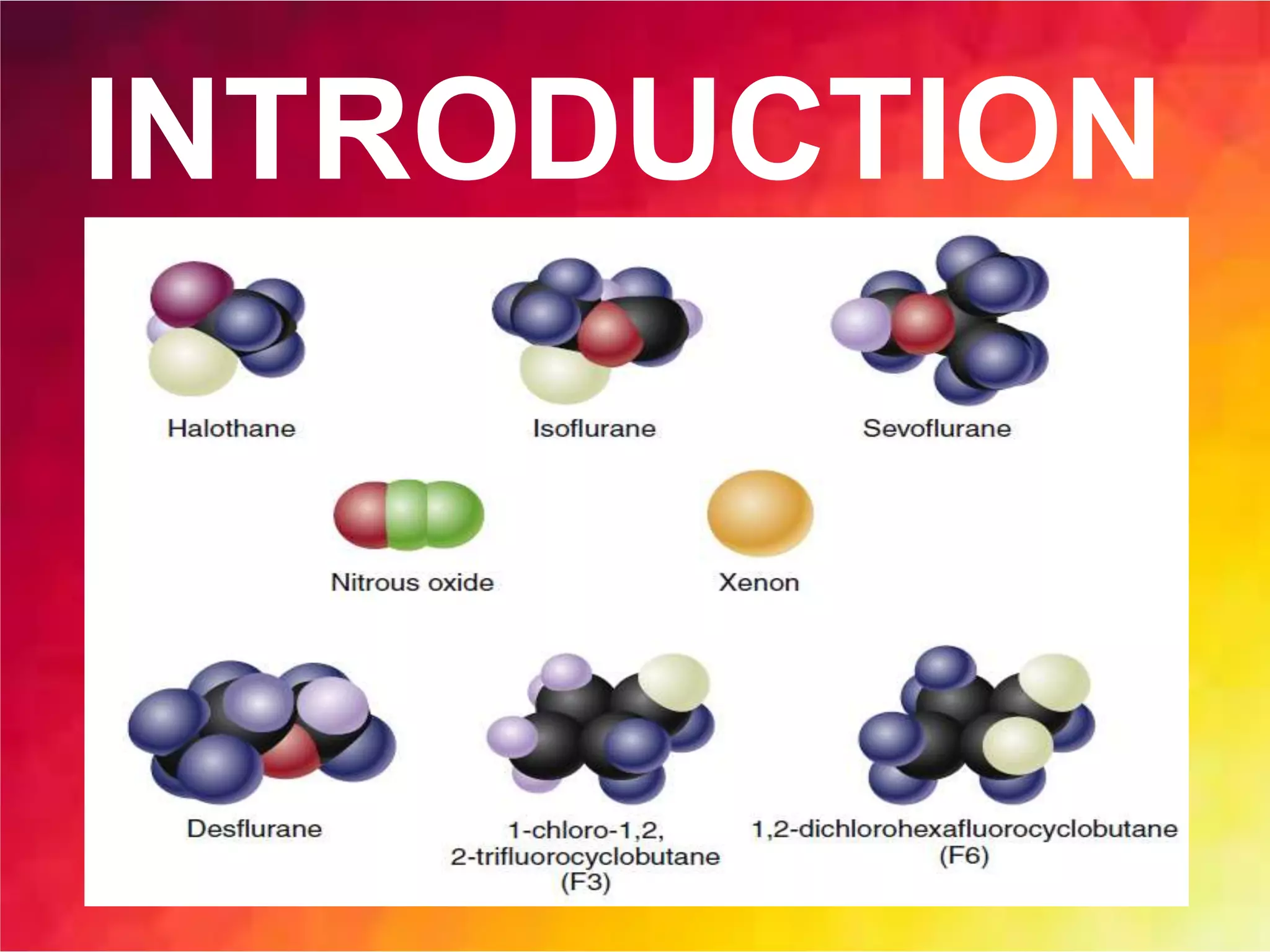
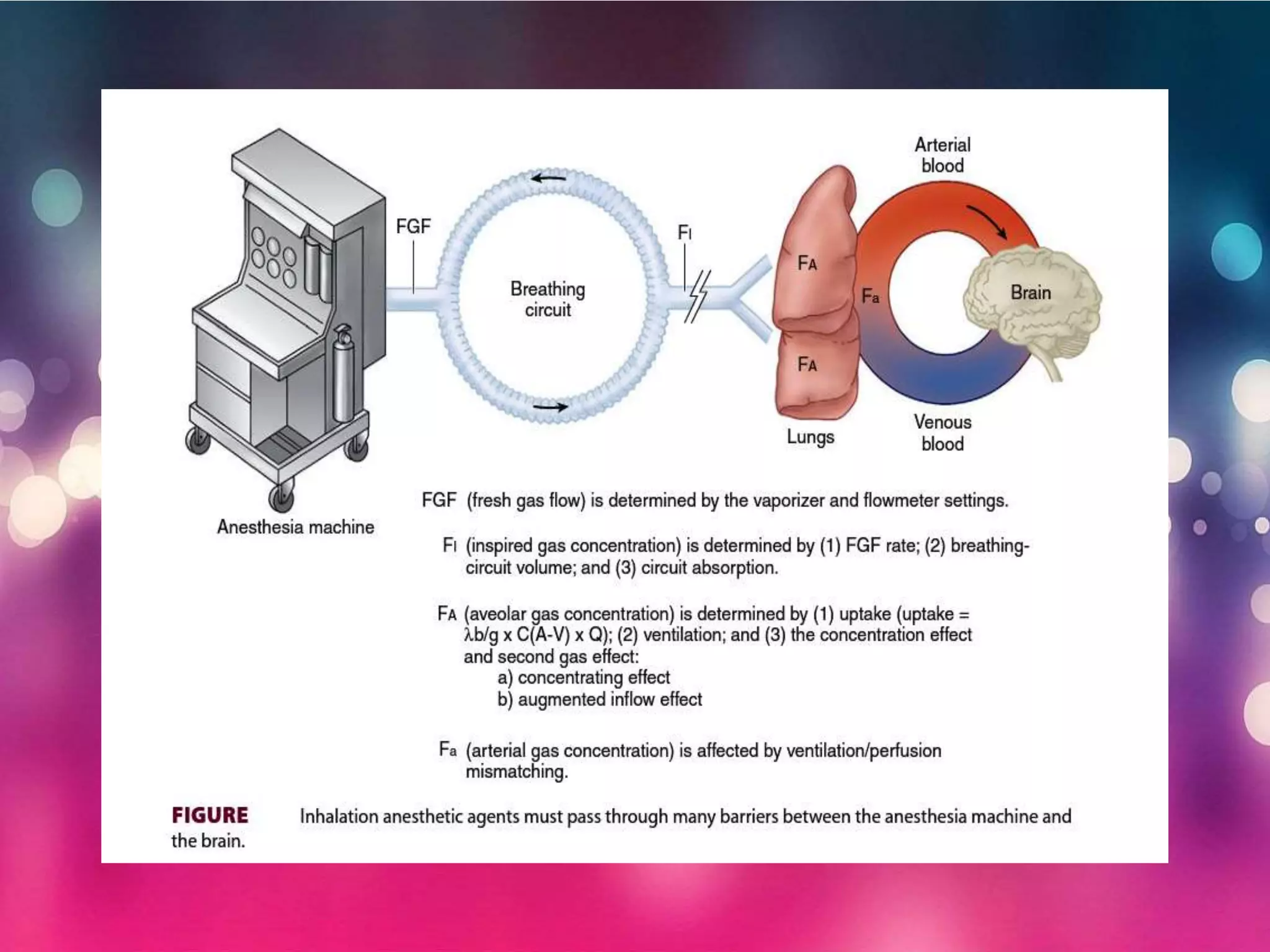
Inhalational anesthetics are commonly used for general anesthesia. They are administered via inhalation and produce unconsciousness by reaching specific concentrations in the central nervous system. Their uptake and distribution throughout the body depends on factors like solubility, alveolar ventilation, and cardiac output. Once inhaled, the anesthetic must pass through the lungs and bloodstream before equilibrating between tissues. The potency of different anesthetics is measured by their minimum alveolar concentration, which prevents movement in response to surgery in 50% of patients.







![FA
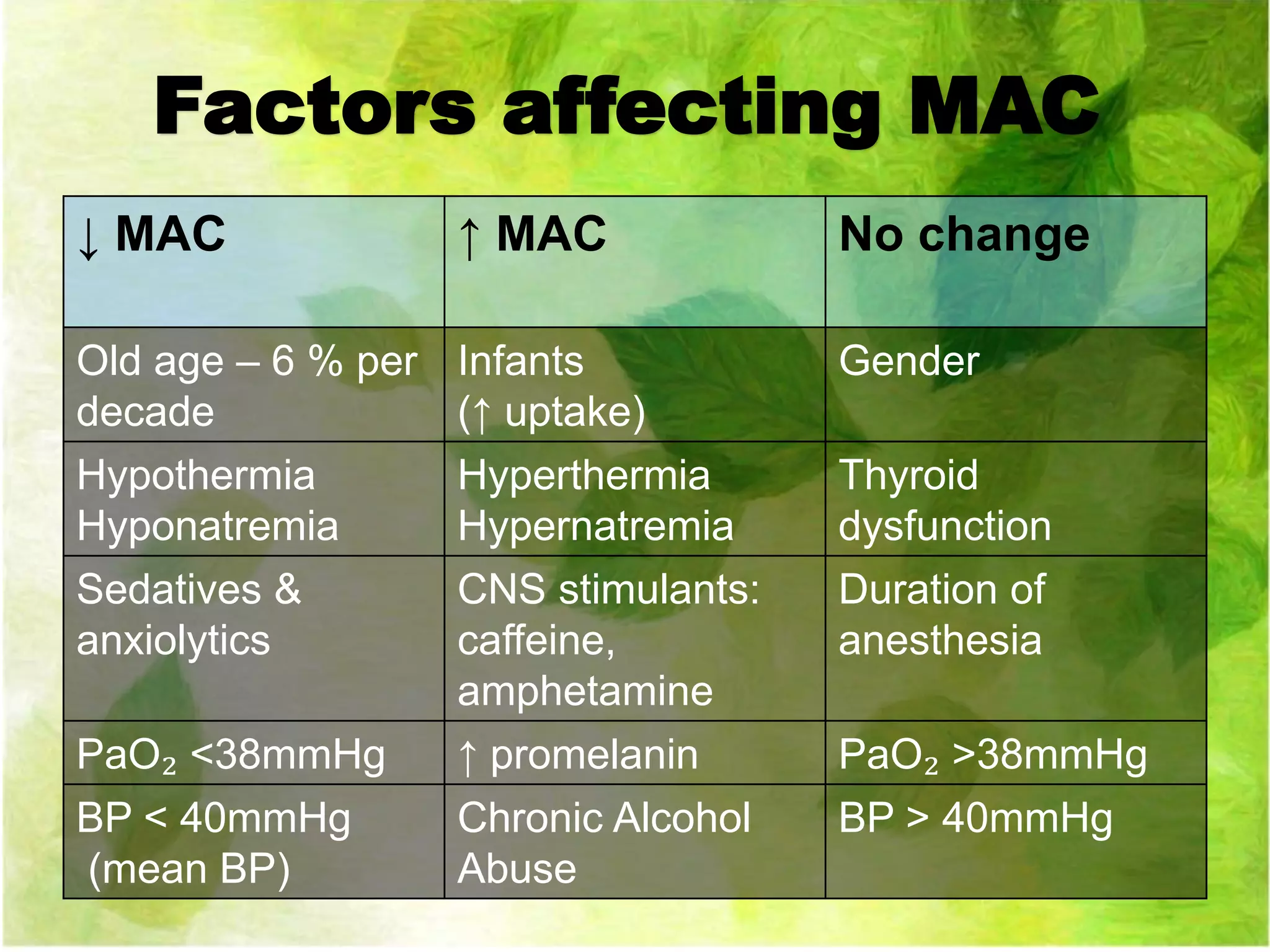
MAC
FD
FI
Ventilation
FA/FI
Time
constant
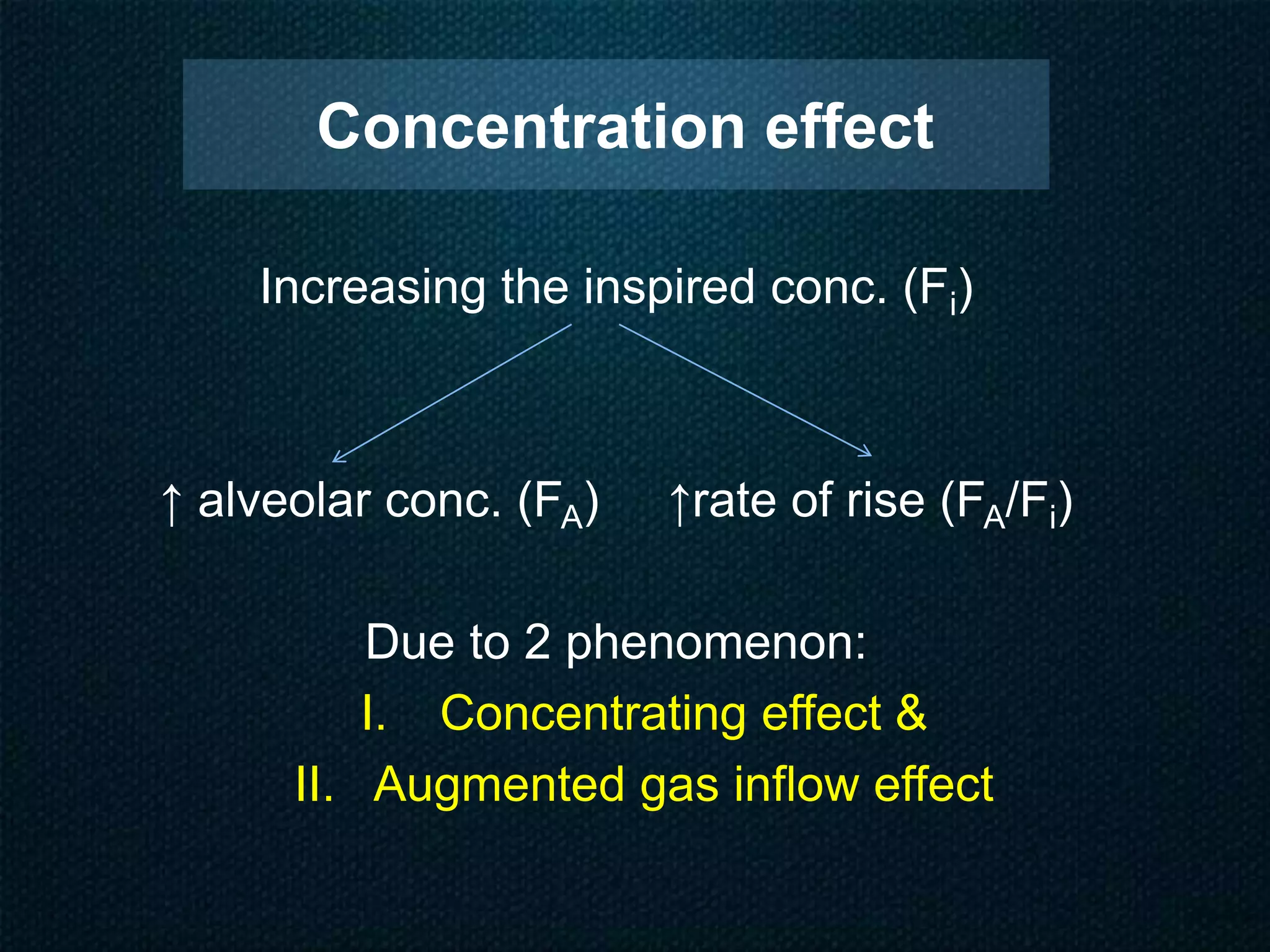
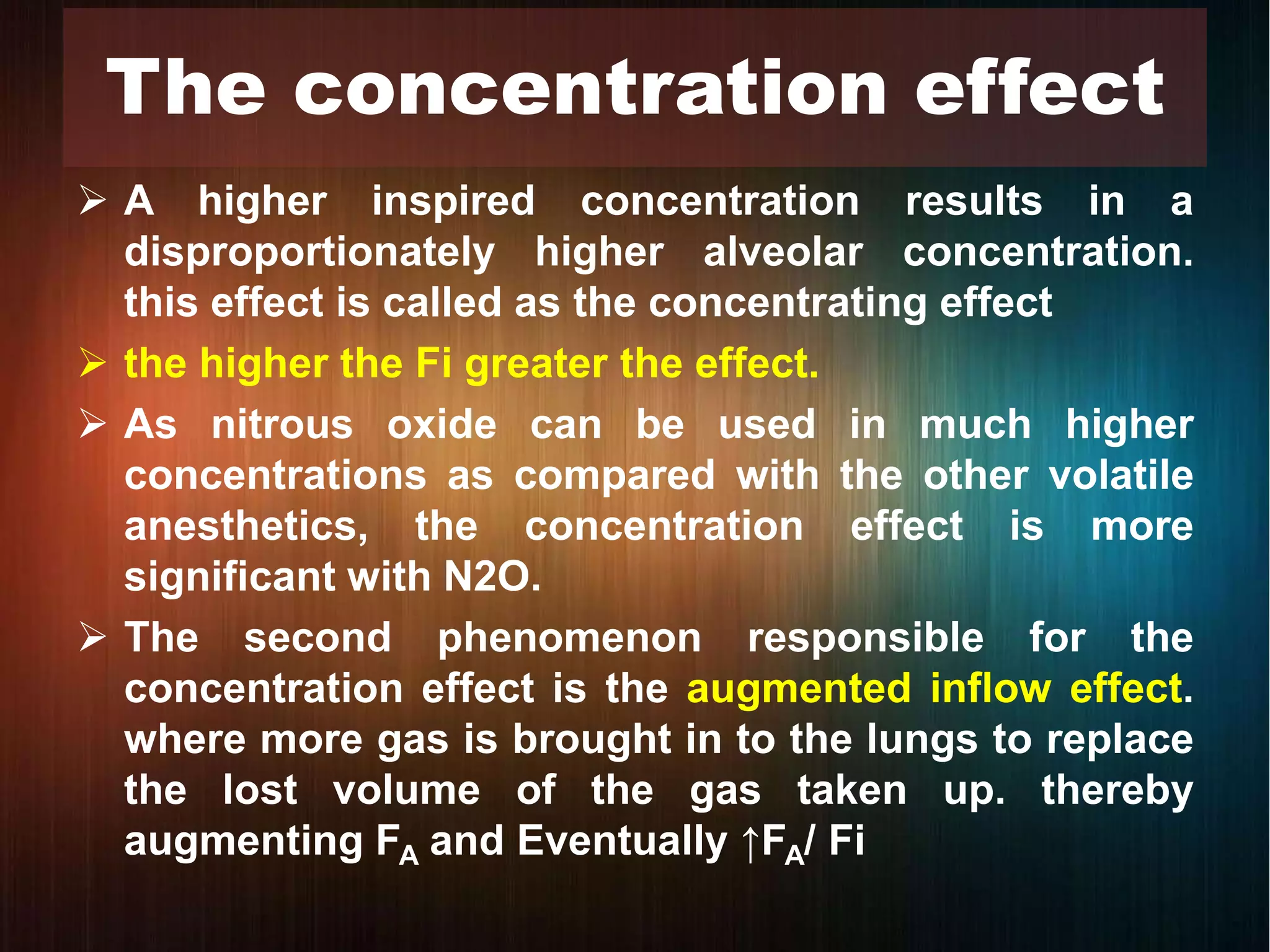
Concentration and
second gas effects
λB/G
CO
PA - PV
VRG
λT/B
Tissue blood flow
[Parterial - PTissue]
Time
constant
Brain Partial
pressure drives
depth of
anesthesia
Equilibrates](https://image.slidesharecdn.com/uptakeandsistributionofinhaledanesthetic-170601153634/75/Uptake-and-distribution-of-inhaled-anesthetic-10-2048.jpg)











![ Solubility is defined in terms of the partition coefficient
Partition coefficient is the ratio of the amount of
substance present in one phase compared with
another, the two phases being of equal volume and in
equilibrium
[λB/G = CB /CG ]
Solubility Capacity of blood & tissue
longer it takes to saturate at given rate
Slower the rate of rise of FA/ Fi](https://image.slidesharecdn.com/uptakeandsistributionofinhaledanesthetic-170601153634/75/Uptake-and-distribution-of-inhaled-anesthetic-27-2048.jpg)