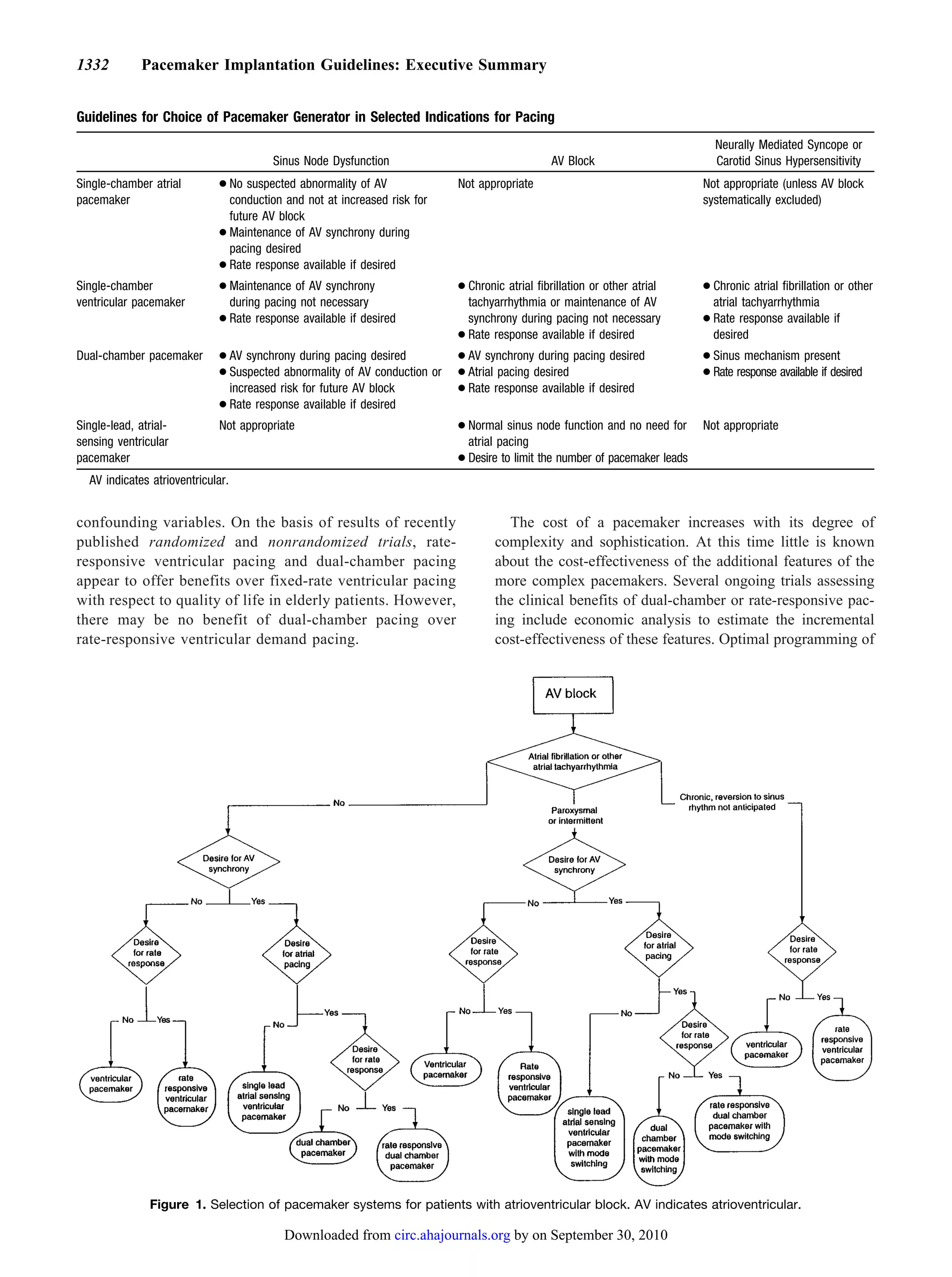
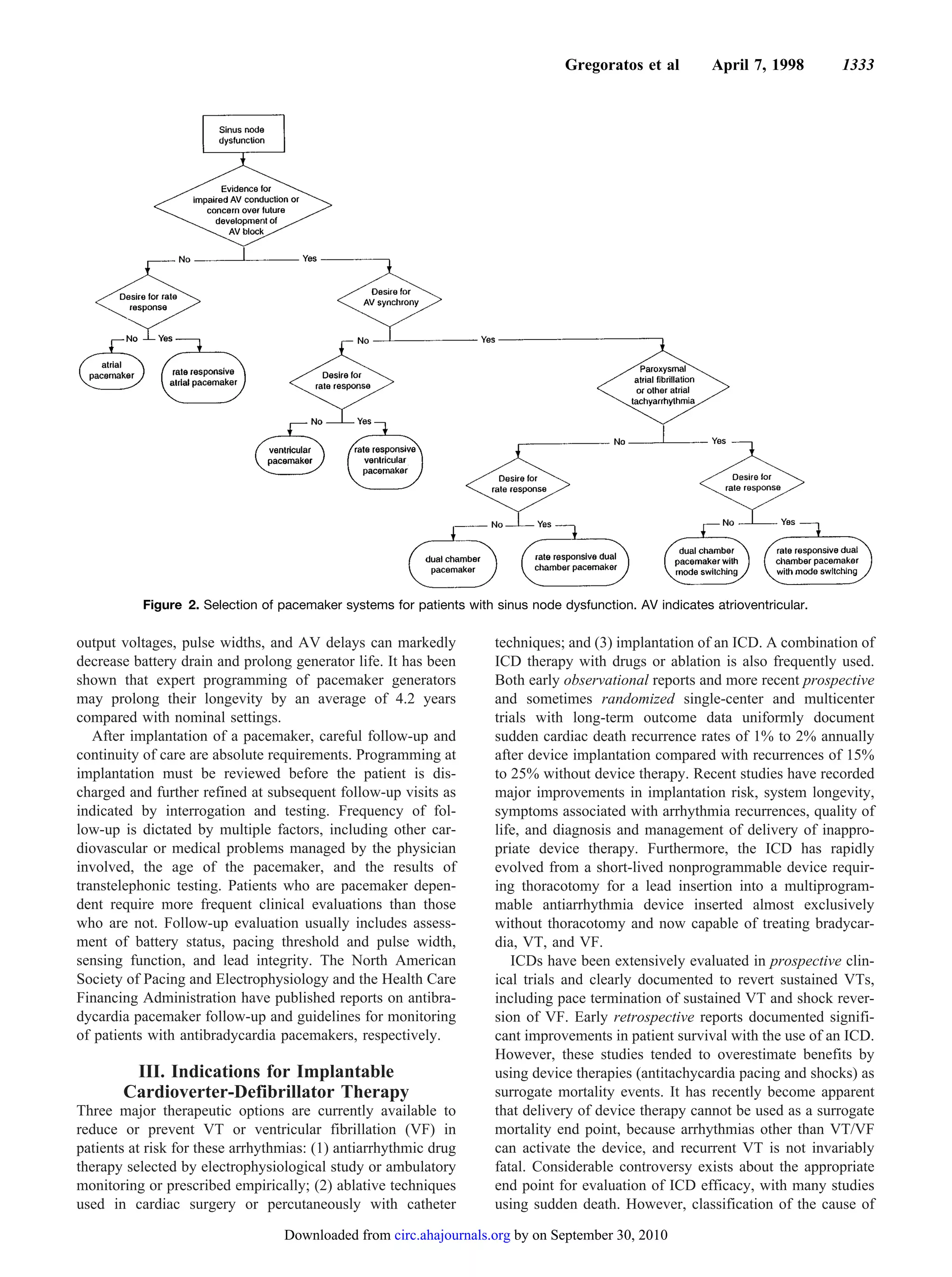
The ACC/AHA guidelines for cardiac pacemaker and antiarrhythmia device implantation were revised based on advances in technology and new clinical evidence. The guidelines provide evidence-based recommendations (level A, B, C evidence) on appropriate patient selection and device optimization. Indications for implantation were expanded based on studies showing risks of untreated bradyarrhythmias and benefits of pacing. The guidelines emphasize the importance of adequate long-term follow-up to ensure devices provide ongoing clinical benefit.