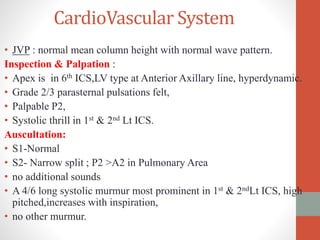
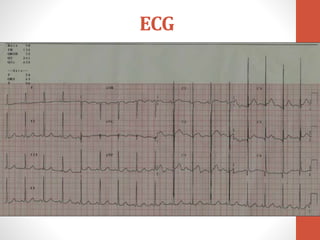
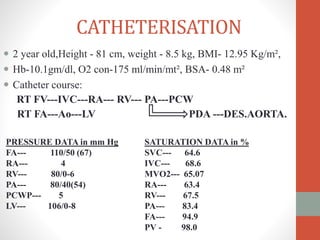
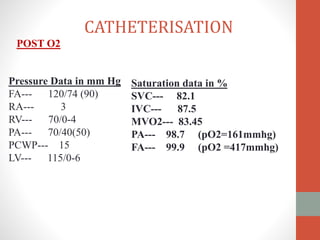
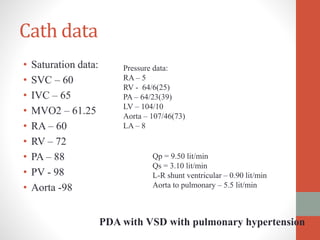
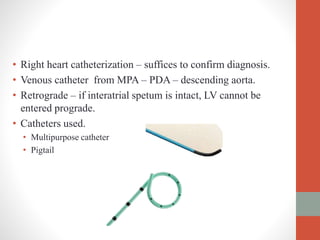
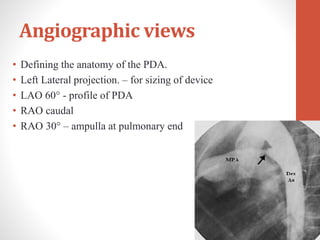
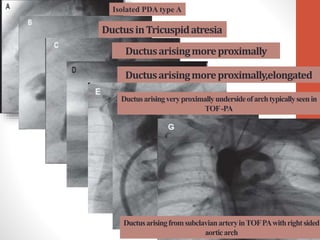
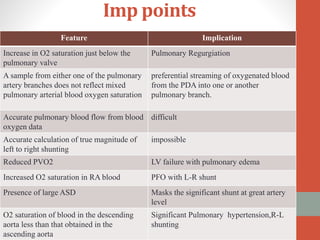
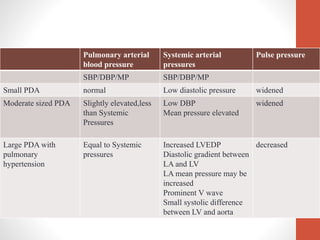
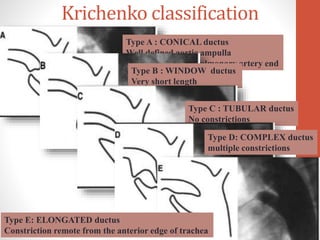
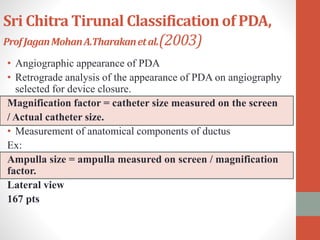
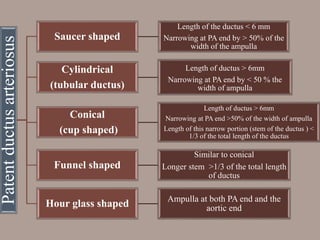
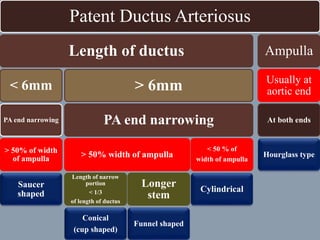
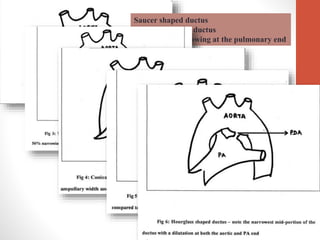
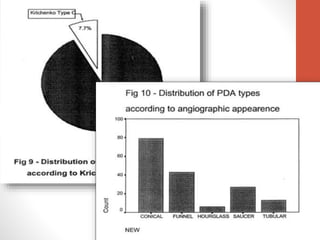
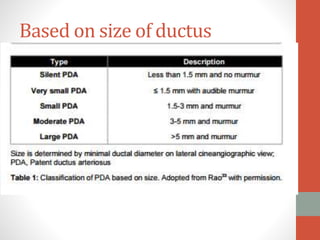
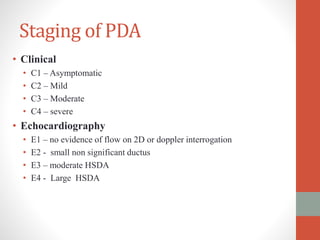
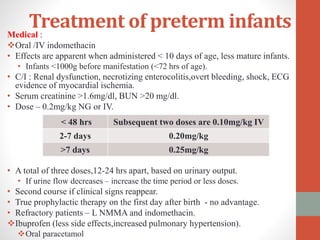
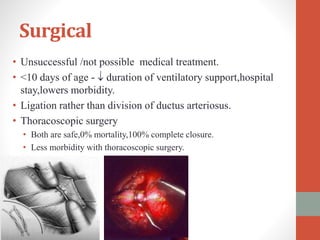
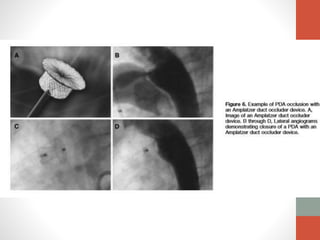
This document discusses the case of a 2 year old male child presenting with recurrent lower respiratory tract infections. On examination, a systolic murmur was heard. Echocardiogram showed a patent ductus arteriosus (PDA) of size 8mm with left to right shunting. Cardiac catheterization found a Qp/Qs ratio of 1.83, confirming a left to right shunt. Post oxygen, the Qp/Qs ratio increased to 2.94, and PVR decreased, indicating reactivity. The document then discusses two other cases and provides information on indications for catheterization in PDA, angiographic views, classifications of PDA, and factors affecting shunting through a PDA