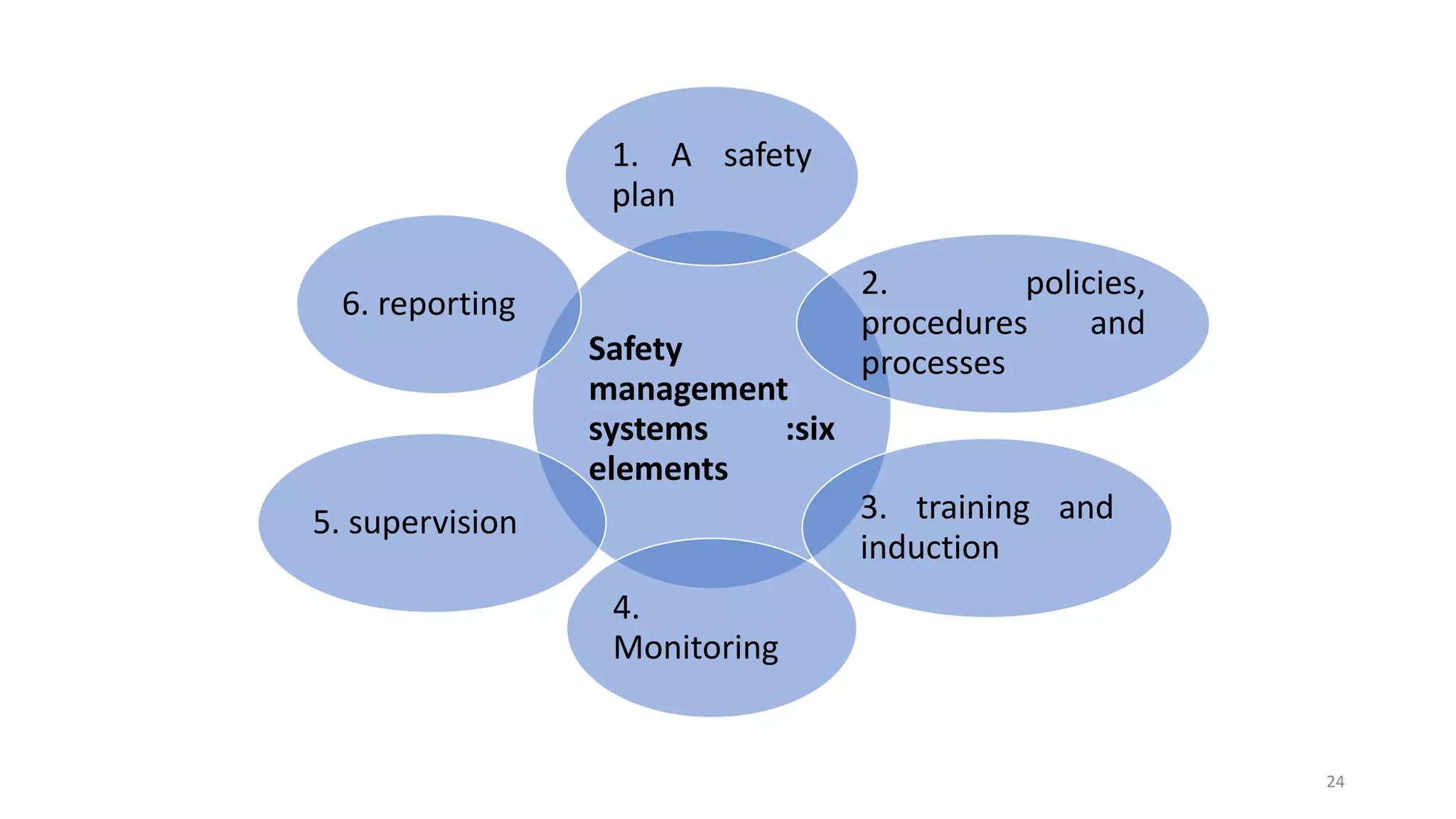
This document discusses hazard and risk management in factories. It covers the Factory Act of 1948 and its provisions regarding health, safety, welfare, and working hours. It describes definitions related to factories and objectives of the Factory Act. Key elements of the Act include provisions for hazardous processes, restrictions on employment of women and children, and annual leave. Accident prevention fundamentals and elements of an effective safety program and management system are also outlined.