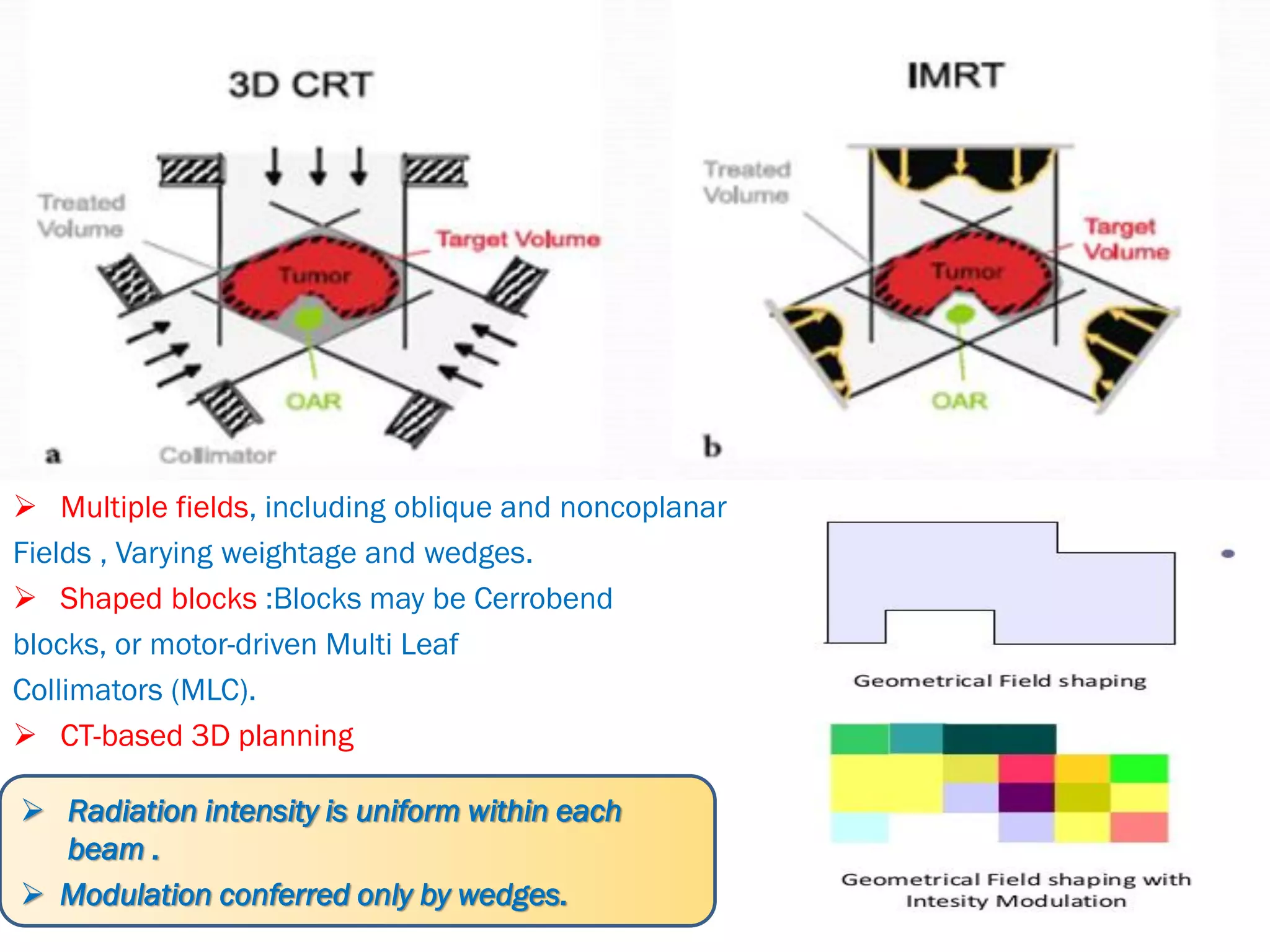
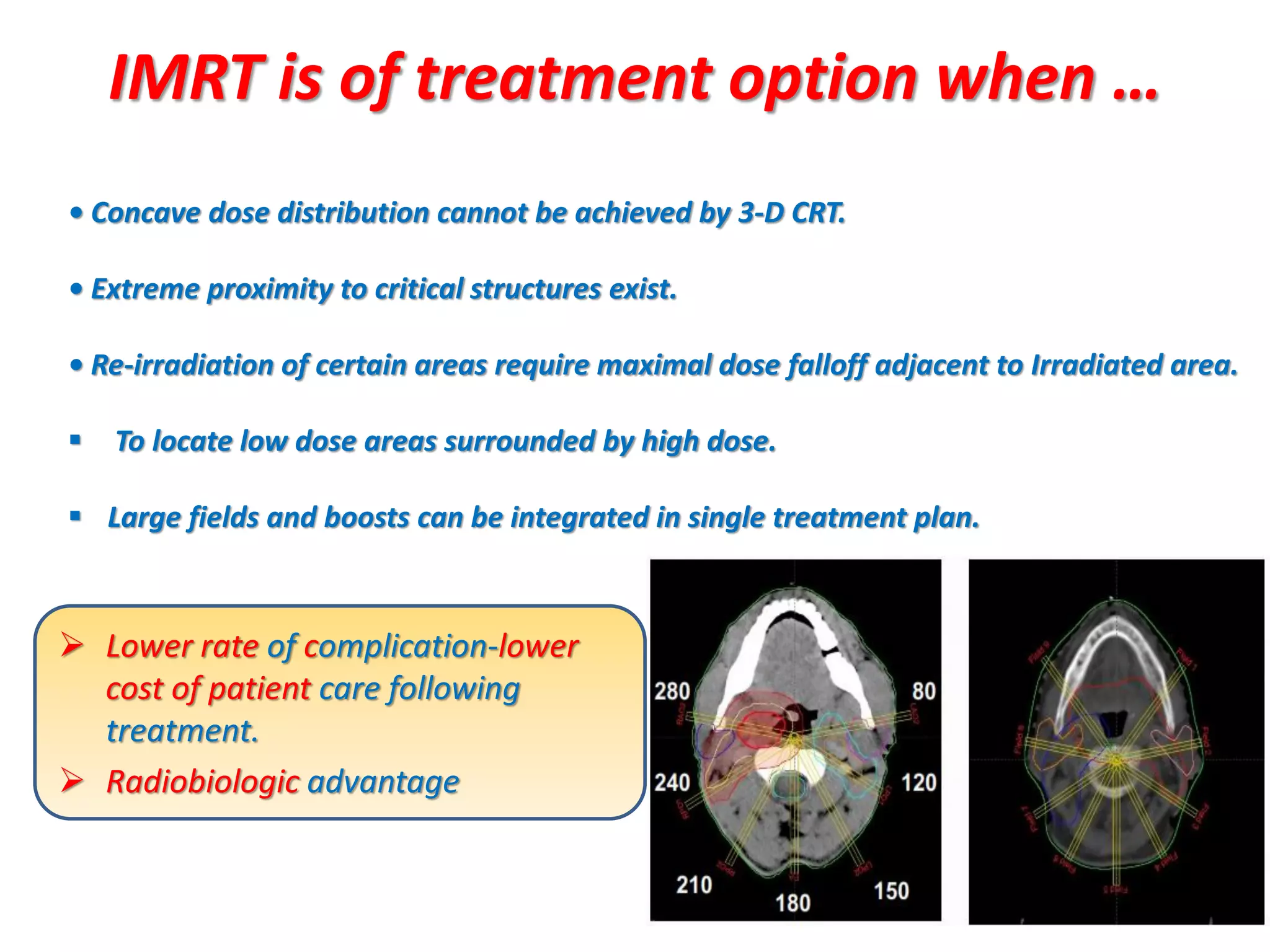
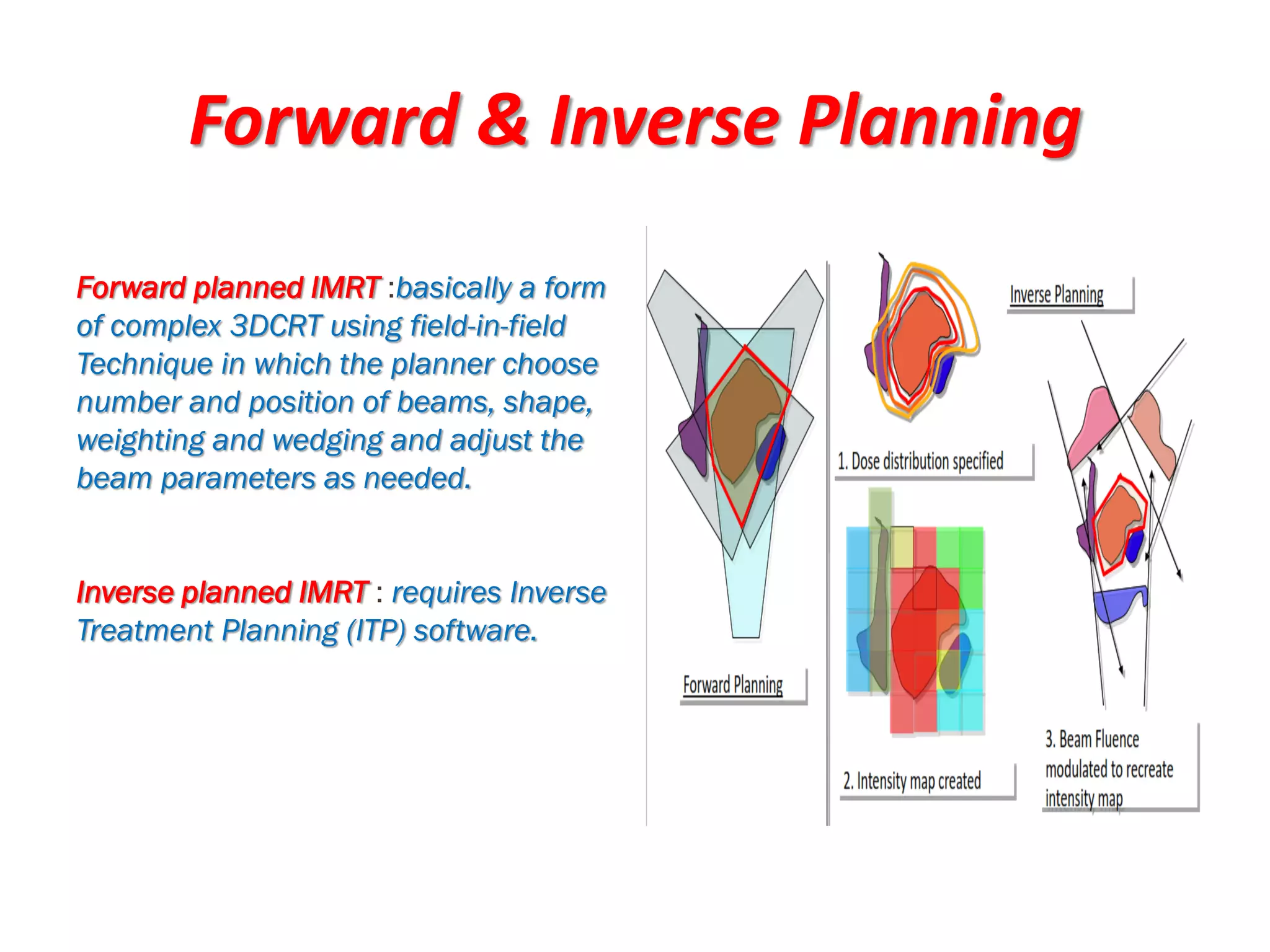
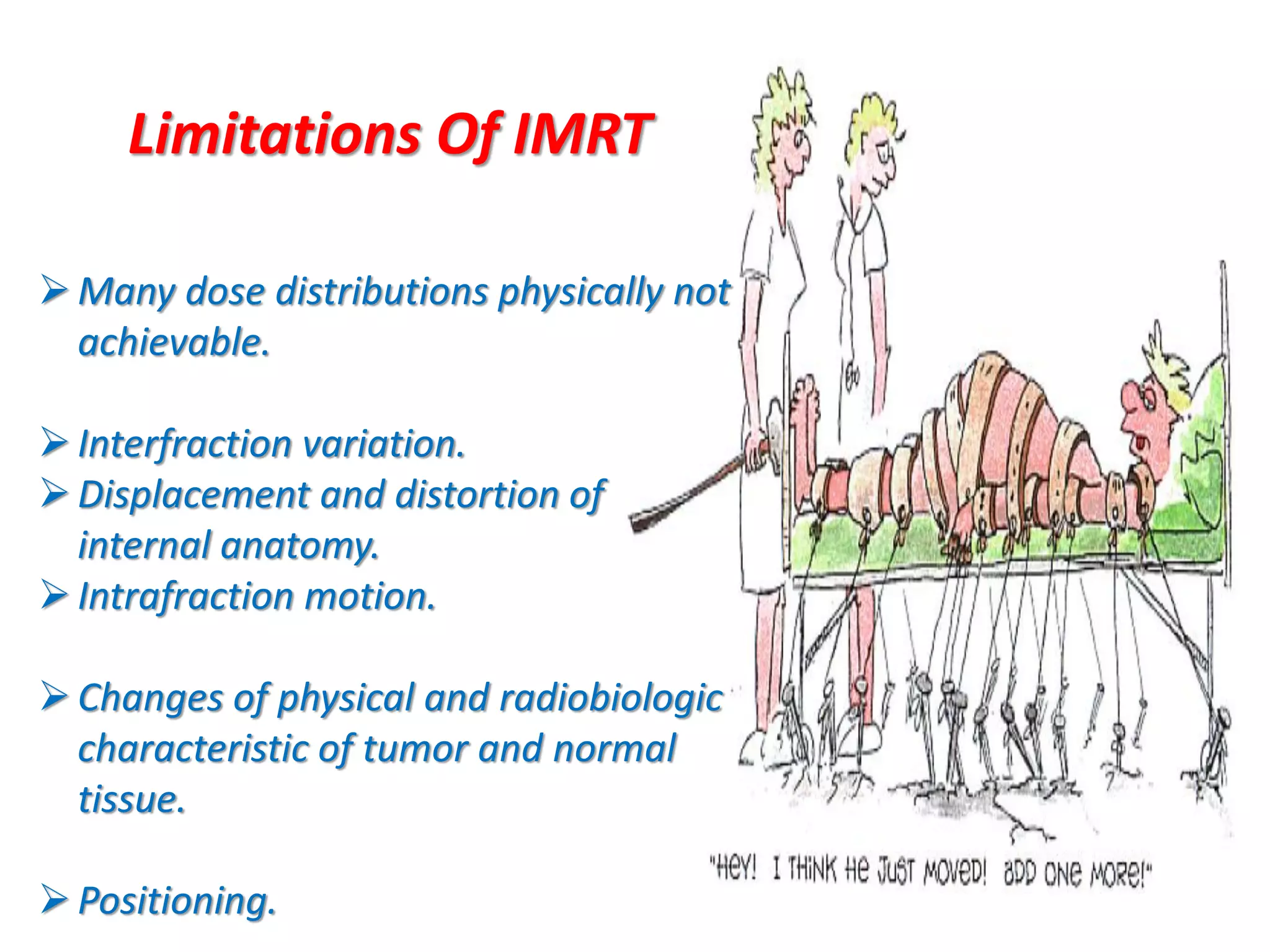
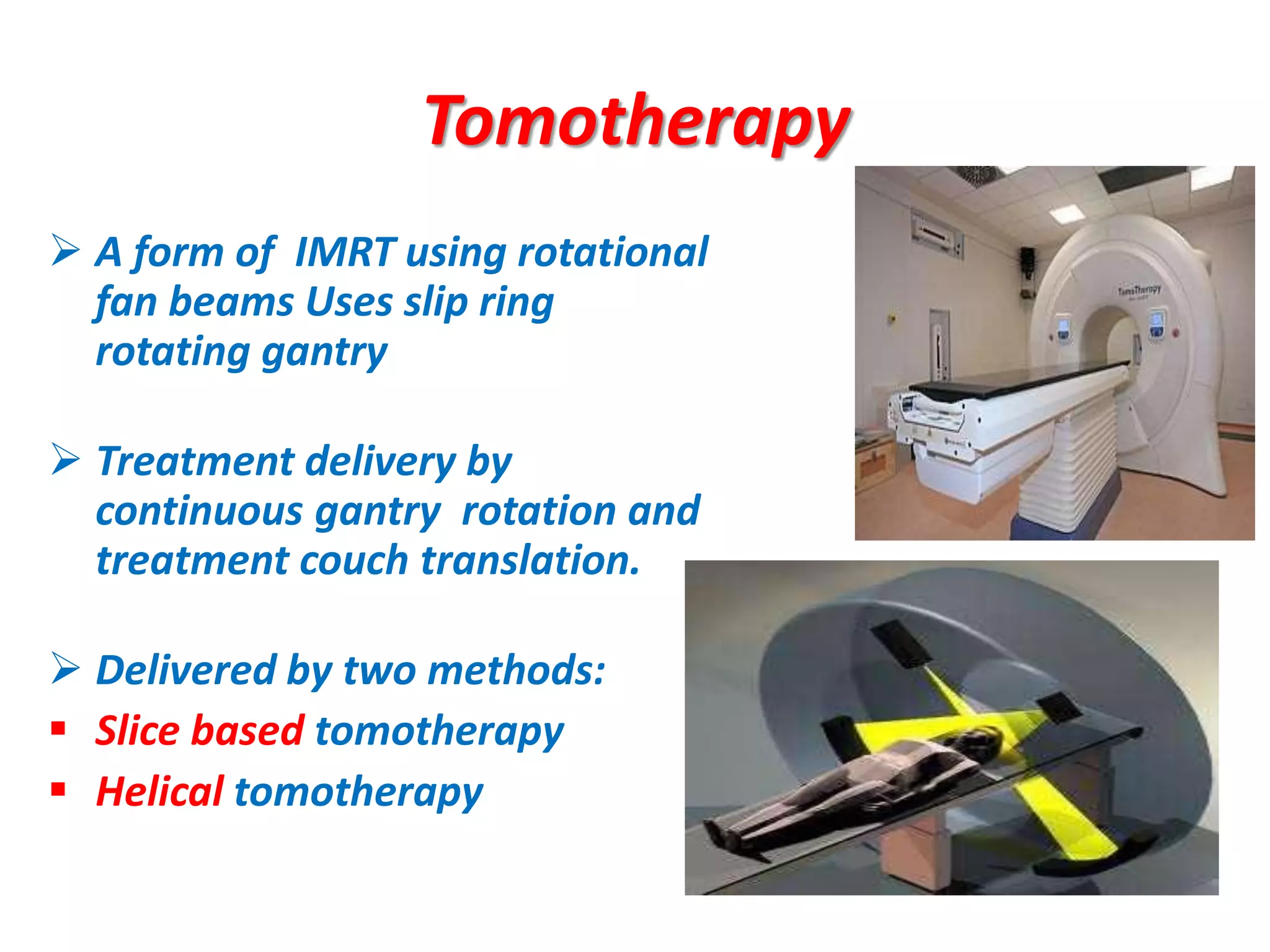
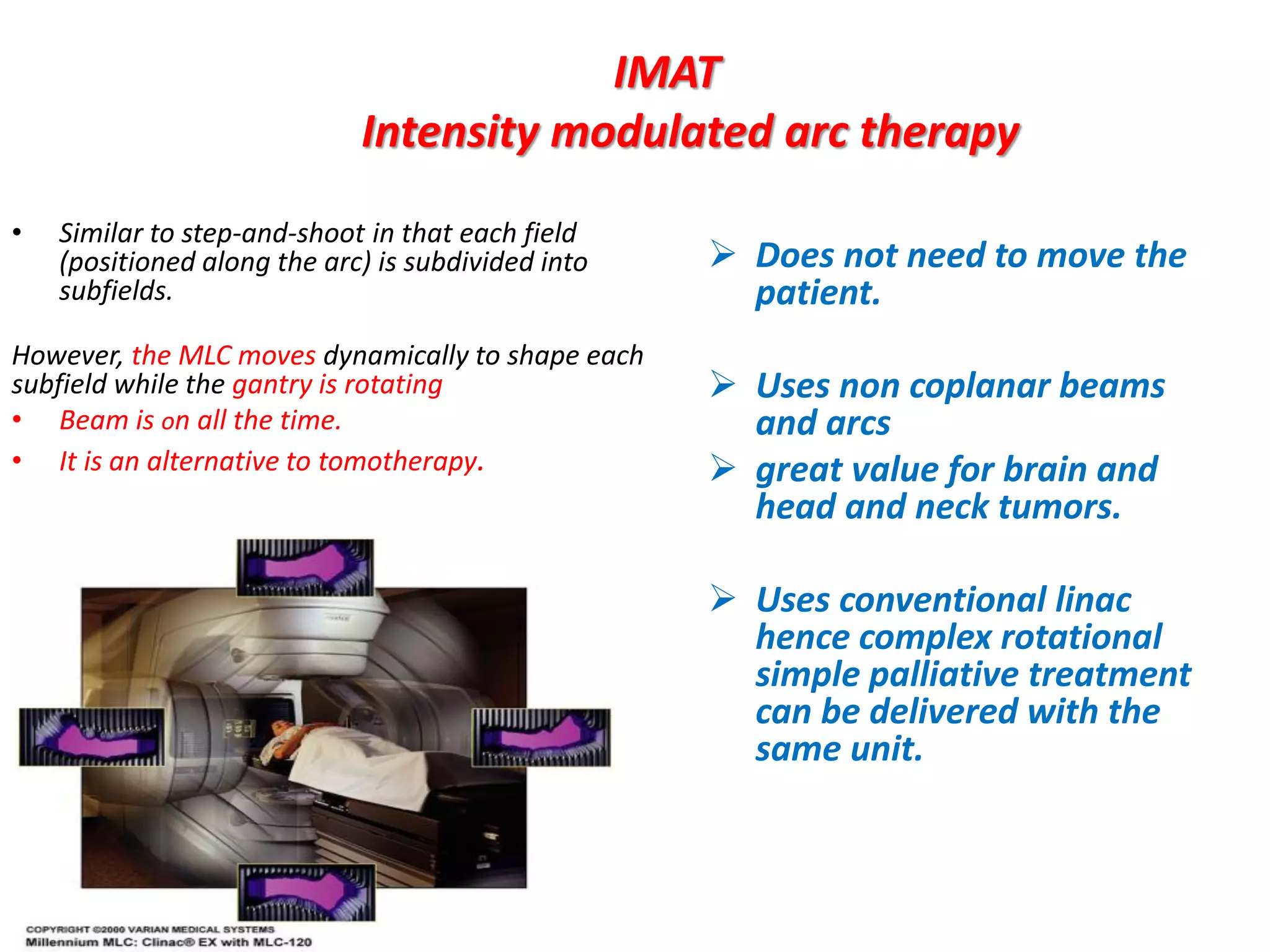
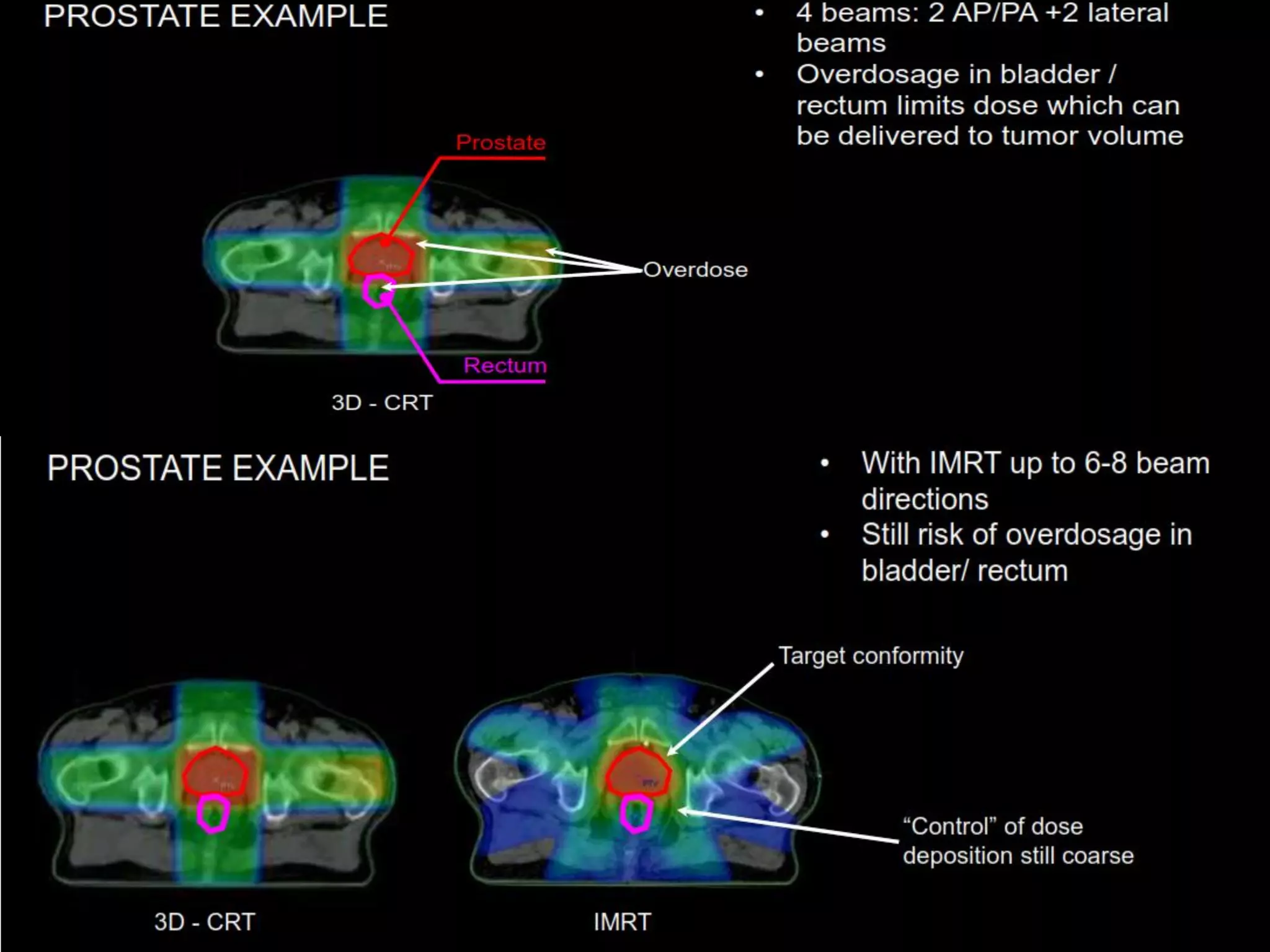
1. The document discusses various aspects of intensity-modulated radiation therapy (IMRT) planning and delivery, including the use of inverse planning, optimization objectives and constraints, and different delivery methods like static field, dynamic field, tomotherapy, and VMAT.
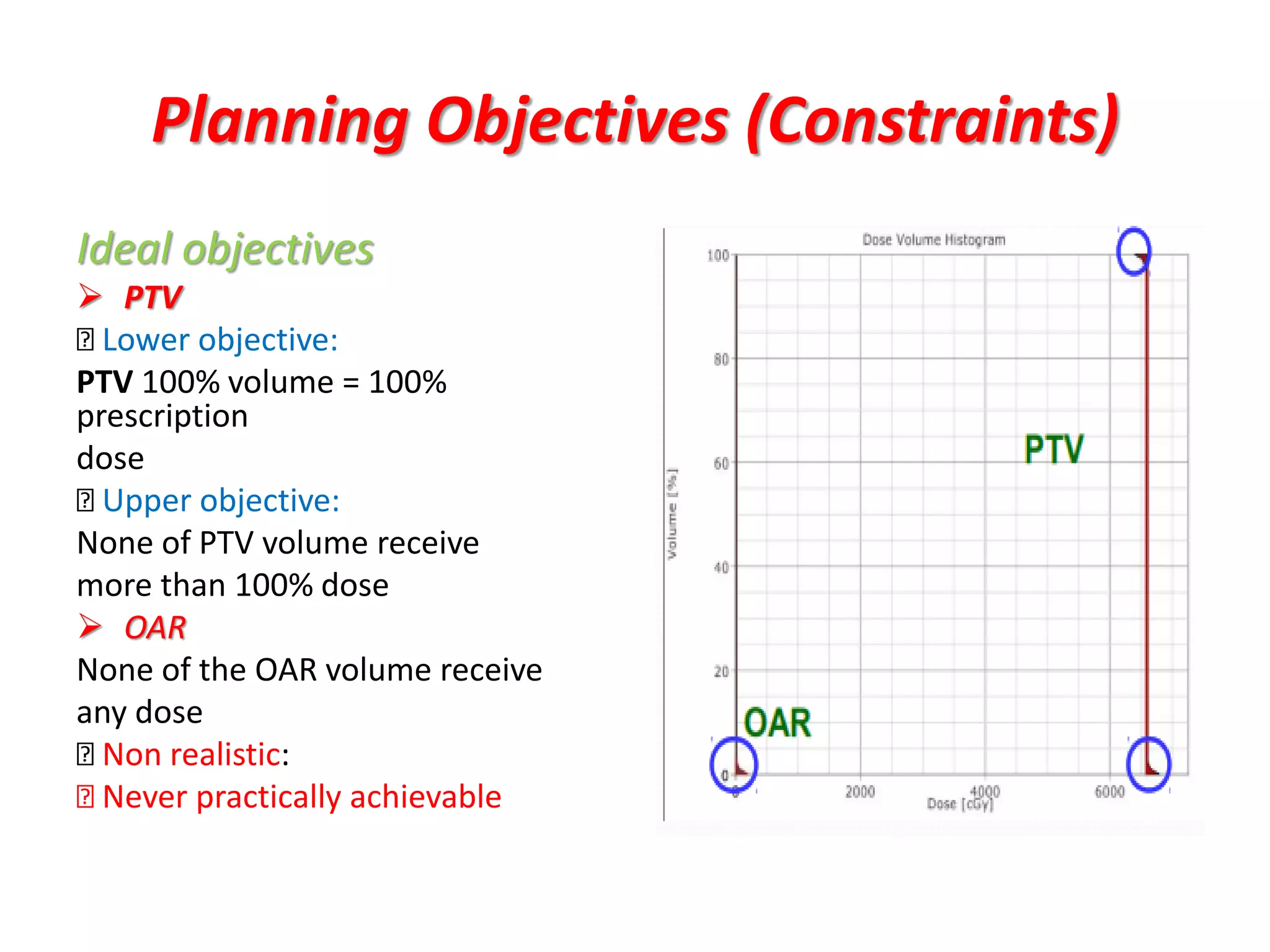
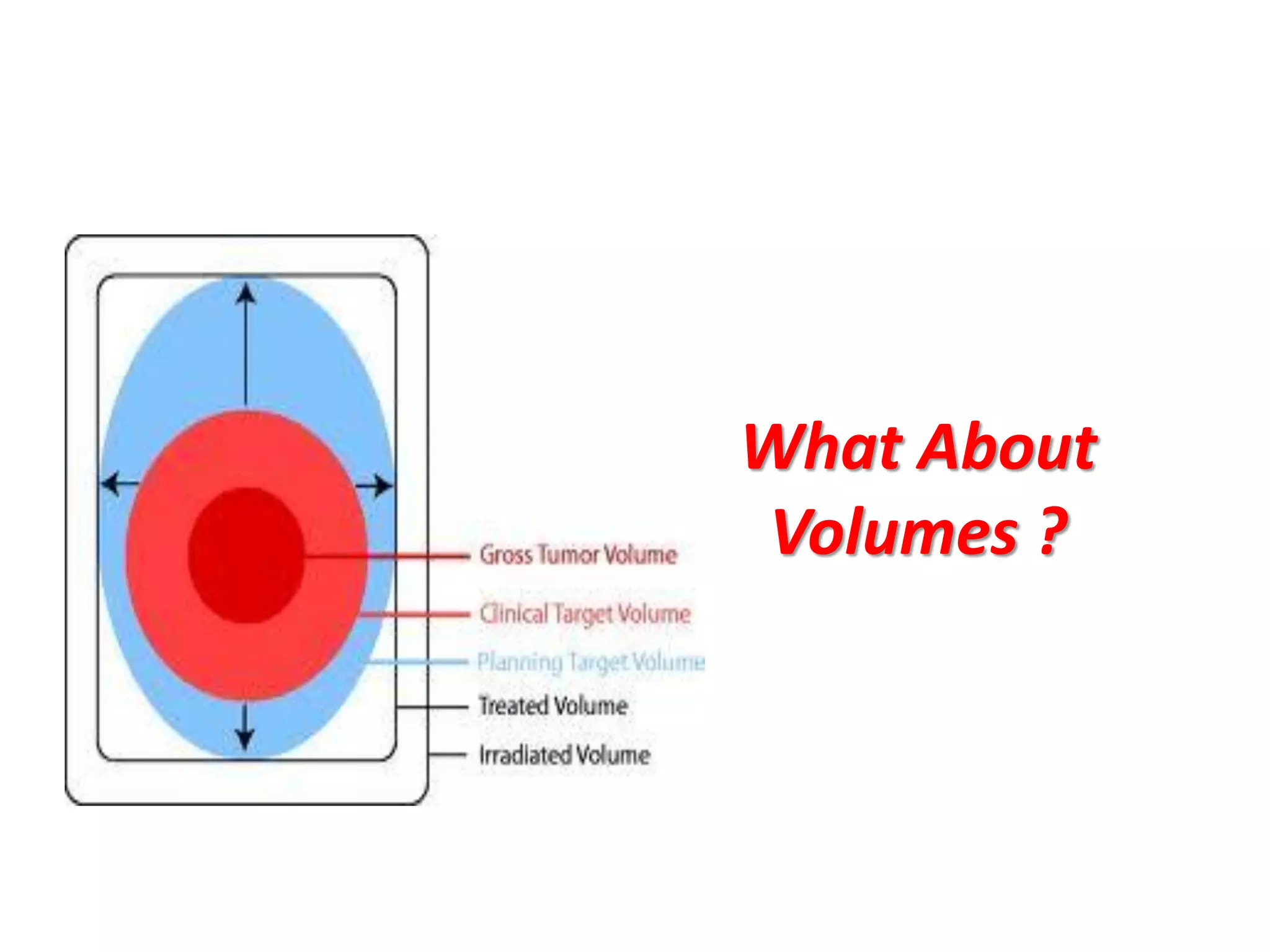
2. It also discusses treatment volumes defined in ICRU 83 like gross tumor volume, clinical target volume, planning target volume, and organ-at-risk volumes. The document emphasizes using dose-volume histograms to specify dose rather than a single point.
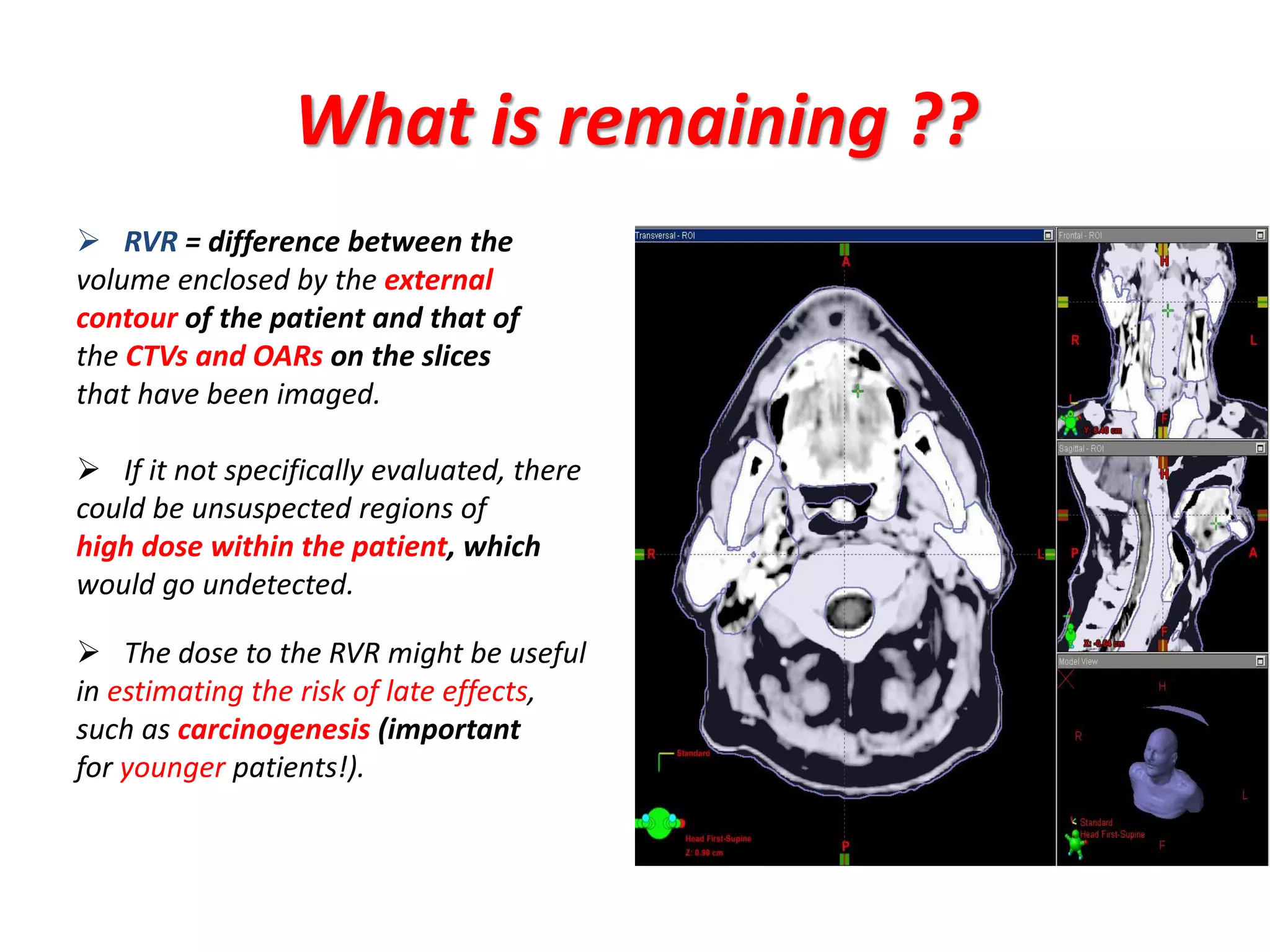
3. Challenges with overlapping treatment volumes and the importance of evaluating the remaining volume at risk are also covered.