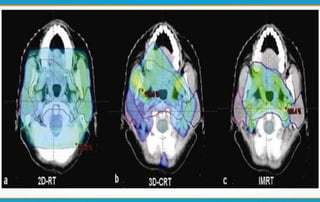
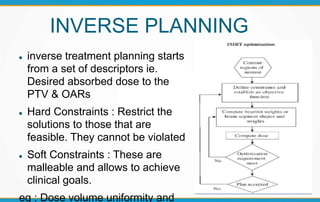
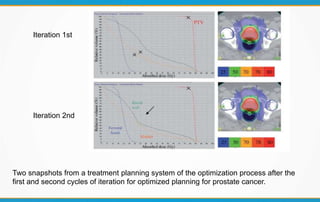
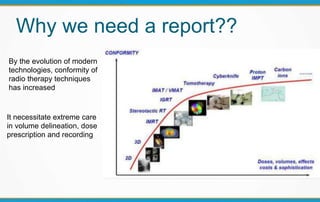
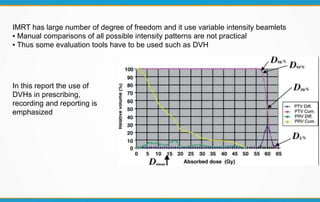
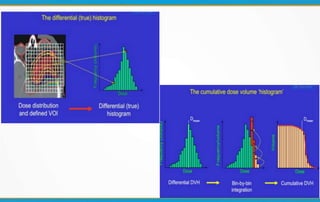
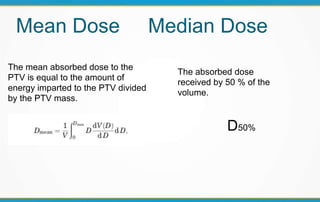
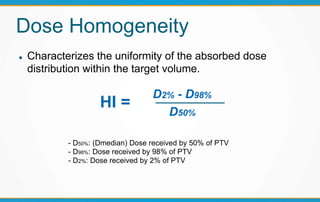
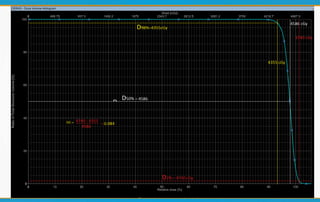
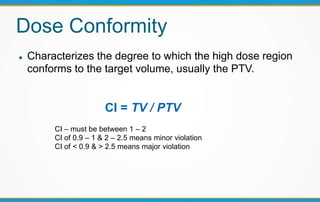
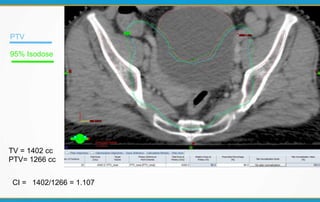
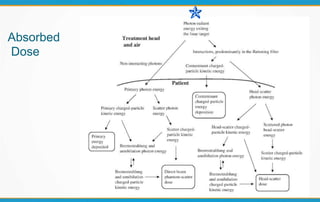
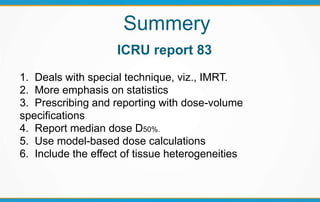
1. ICRU Report 83 provides guidelines for prescribing, recording, and reporting intensity-modulated radiation therapy (IMRT). It emphasizes using dose-volume histograms and statistics like median dose to describe dose distributions.
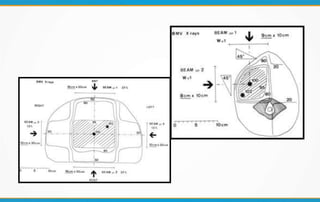
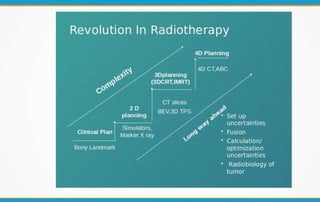
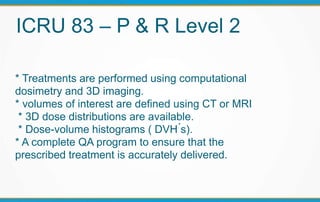
2. The report outlines three levels of prescribing and reporting with increasing complexity. Level 1 involves basic 2D dose distributions while Level 3 incorporates more advanced metrics like tumor control probability.
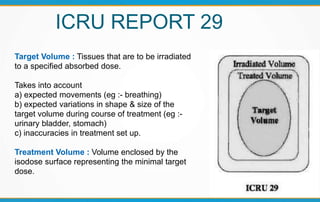
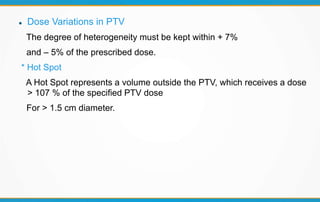
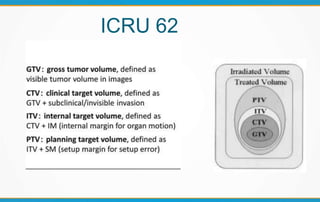
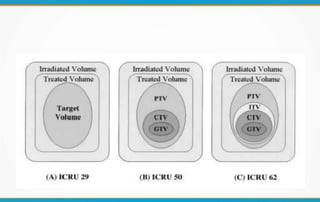
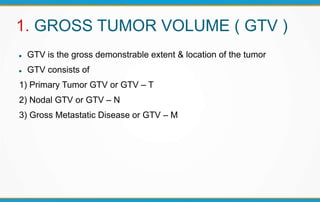
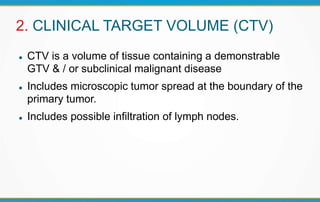
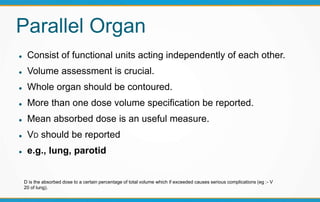
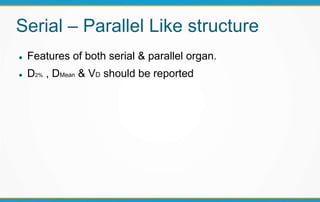
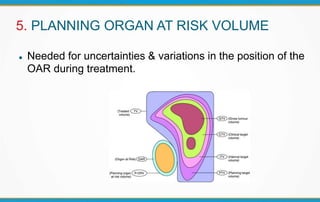
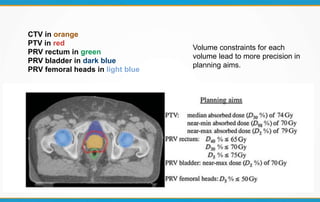
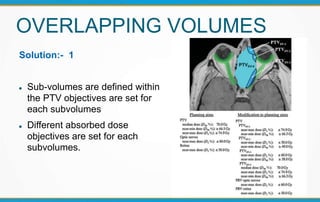
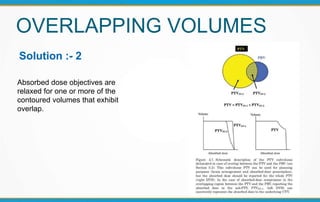
3. Key volumes discussed include gross tumor volume, clinical target volume, planning target volume, and organs at risk. The report standardized how to account for uncertainties and patient motion when defining these volumes.