This document discusses intensity-modulated radiation therapy (IMRT) planning. It describes how IMRT allows for more conformal radiation dose distributions compared to 3D conformal radiation therapy by modulating the fluence or intensity of the radiation beams. This capability of IMRT is particularly advantageous for sites with concave target volumes or where sensitive structures are located very close to the target, like for prostate cancer treatment. The document covers the historical development and technical aspects of IMRT planning and optimization methods as well as examples of clinical applications.


















![distance moved per time elapsed. Given the ability to precisely
control the effective velocity of all the MLC leaves, changing
the effective velocities of the leading leaves and the trailing
leaves determines the accumulated fluence delivered for each
beamlet along the leaf-pair track. The beamlet intensity is
determined by t, the difference in time (in units of MU) at
which the leading leaf crosses the beamlet position and the
time (in units of MU) at which the trailing leaf crosses the
beamlet position. This MU difference determines the beamlet
fluence value and is directly related to the dosimetry of the
treatment.
9.17.3.2.1 Leaf-pair speed optimization
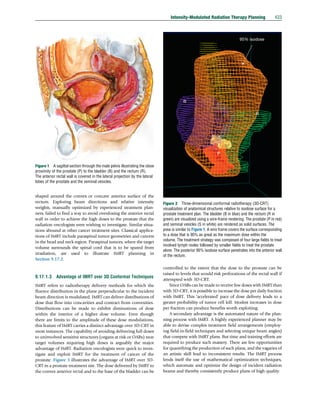
What algorithm will best compose a dynamic LT schedule for a
fluence profile? The ideas behind the algorithm can be dis-
cussed with the aid of Figure 19. Figure 19(a) depicts a fluence
profile against position. This simple arbitrary example contains
three maxima and two minima. Thus, it has six regions deter-
mined by the sign of the gradient of the profile. The modulated
fluence is to be created by a schedule for leaves moving from
left to right with a leading leaf B on the right moving with
velocity VB and a trailing leaf A on the left moving with velocity
VA. The mathematical derivations of the leaf velocities, VA(t)
and VB(t), must be such that the MU delivered between the
time leaf B reaches a position and opens it to the receipt of
radiation and the time leaf A reaches the position and shuts off
the radiation to that point is equal to the beamlet intensity for
that position. The first positive gradient region is similar to a
wedged field with a complex shape created by a gap increasing
between the leaves. It could be delivered with the trailing leaf
being stationary and the velocity of the leading leaf modulated
to form the beam shape. However, the next region has a
negative gradient and must be delivered by a closing gap. The
leading leaf B must therefore race to the position of the first
maximum with maximum velocity, Vmax, so that it can partic-
ipate in a closing gap beginning at that point. The regions of
the graph having negative slope can be rotated about the
vertical (Figure 19(b)) and shifted (Figure 19(c)) to maintain
the same opening time t as the original profile but allow for
closing gaps. The resulting figure can then be skewed with a
slope that corresponds to the maximum leaf velocity
(Figure 19(d)). The graphic operations can be translated into
mathematical operators from which the leaf velocities can be
derived (Xing et al., 2005). The leaf velocities for leaf A, VA, and
leaf B, VB, as a function of the leaf position for the dynamic
leaf sequence were originally derived independently by sev-
eral investigators (Dirkx et al., 1998; Spirou and Chui, 1994;
Svensson et al., 1994).
Y gradient VA VB
Positive Vmax/[1þVmax (dYdx)] Vmax
Negative Vmax Vmax/[1ÀVmax (dYdx)]
where dY/dx is the gradient of the fluence profile. The numer-
ical result for our example in Figure 19 using the equations
earlier is depicted in Figure 20.
9.17.3.2.2 Special quality assurance
The actions sufficient to insure the safe and accurate perfor-
mance of an IMRT treatment system fall into two overlapping
18 50 MU
19
20
21
22
18 60 MU
19
20
21
22
18 70 MU
19
20
21
22
18 80 MU
10 MU
20 MU
30 MU
40 MU
19
20
21
22
18 90 MU
19
20
21
22
18 100 MU
19
20
21
22
18 110 MU
19
20
21
22
18
19
20
21
22
18
19
20
21
22
18
19
20
21
22
18
19
20
21
22
Figure 18 The leaf aperture sequence produced by the matrix method. The MU identify the order of the sequence. The leaf-track numbers are
given to the right of each instance of the sequence. Note that the gaps sweep from right to left. The sequence of gap widths and locations are the same as
that depicted for the step-and-shoot sequence given in Figure 16.
Intensity-Modulated Radiation Therapy Planning 451](https://image.slidesharecdn.com/2c9eb4f4-2888-4a25-884c-51d7148dc56c-151007162026-lva1-app6892/85/IMRT-21-320.jpg)











![9.17.5.2.2 Navigating the Pareto surface
We assume for now that we have a set of Pareto-optimal
treatment plans that approximate the Pareto surface. Methods
to generate such plans are outlined in the succeeding text in
Section 9.17.5.2.3. The set of plans forms a database of opti-
mized IMRT plans; each plan is therefore referred to as a
database plan. Given a set of Pareto-optimal database plans,
the planner is to be provided with methods to explore the
trade-off space. A naive way to approach this consists in letting
the treatment planner choose one of the database plans. How-
ever, it is desirable to explore trade-offs in a continuous fash-
ion. To that end, not only database plans themselves are
considered but also their combinations.
9.17.5.2.2.1 Convex combinations of database plans
We assume that a treatment plan is defined through the fluence
map x. Given two treatment plans with fluence maps x1
and x2
,
we can form a convex combination of the two treatment plans
by considering the averaged fluence map
x ¼ q x1
þ 1 À qð Þx2
which is obtained by averaging the beamlet intensities beamlet
by beamlet, using a mixing parameter qE[0,1]. If x1
and x2
are
Pareto-optimal treatment plans, the convex combination of
two plans is expected to be also a ‘good’ treatment plan.
Since the dose distribution is a linear function of the fluence
map, averaging of the fluence maps corresponds to averaging
the dose distributions of the two plans. To characterize the
quality of the averaged treatment plan, we discuss its location
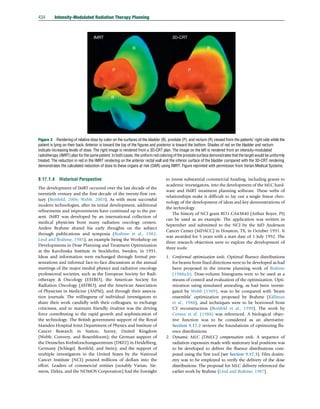
in objective space with respect to the Pareto surface. This is
illustrated in Figure 32: by averaging the objective function
values obtained for the two plans, we obtain points
qfT x1
À Á
þ 1 À qð Þ fT x2
À Á
, qfS x1
À Á
þ 1 À qð Þ fS x2
À ÁÀ
in the two-dimensional objective function space. These points
form a line that connects the two Pareto-optimal treatment
plans (red line in Figure 32). For convex objective functions
fT and fS (which is the case for the commonly used functions
except for DVH objectives), it is known (by definition of a
convex function) that the objective functions evaluated at the
averaged fluence map are smaller than the averages of the
objective values, that is,
fT x1
þ 1 À qð Þx2
À Á
q fT x1
À Á
þ 1 À qð Þ fT x2
À Á
and analogously for the spinal cord. On the other hand, the
average treatment plan is not generally Pareto-optimal. There-
fore, the averaged treatment plan is located in between the true
Pareto surface and the linear approximation (red line), as
indicated by the green dot in Figure 32. Informally speaking,
a convex combination of two treatment plans is expected to be
close to being Pareto-optimal if the Pareto surface is relatively
flat in between the plans being averaged. This idea is reflected
in some of the methods to approximate Pareto surfaces using
as small number of plans, for example, the Sandwich method
discussed in the succeeding text.
For real-world IMRT planning problems, more than two
treatment plans can be combined. If there are M database
plans, the exploration of trade-offs can consider the convex
hull of database plans:
x j x ¼
XM
m¼1
qmxm
,
XM
m¼1
qm ¼ 1
( )
9.17.5.2.2.2 Graphical user interface
In a treatment planning system, the planner has to be provided
with tools to navigate in the convex hull of database plans. In a
practical scenario, the planner may have evaluated a current
treatment plan and would like to improve the treatment plan
regarding one particular objective, say, the mean kidney dose.
The treatment planning system has to provide a user interface
to express this request. Figure 33 shows the multicriteria plan-
ning interface in the RayStation treatment planning system,
distributed by RaySearch Laboratories. Each objective is asso-
ciated with a slider. By moving the slider, the user can request
an improvement of the treatment plan with respect to the
corresponding objective. In the background, the treatment
planning system translates the slider movement into a new
convex combination of database plans (Monz et al., 2008).
Qualitatively, a database plan m that was generated by empha-
sizing the objective corresponding to the slider will be assigned
a higher coefficient qm.
For a high-dimensional trade-off space with many objec-
tives, there may be many ways to achieve this goal. For exam-
ple, reducing the dose to the kidneys can be achieved by
compromising target dose homogeneity or by compromising
the conformity of the dose distribution in the remaining nor-
mal tissue. By locking sliders (visible as the check boxes to the
left of each slider in Figure 33), the user has additional control
over the navigation process. For example, by locking the slider
for target dose homogeneity, the user can request that the
navigation is restricted to treatment plans for which the target
homogeneity is no worse than indicated by the current slider
position.
Convex objectives:
True pareto surface
fT
fT (x1
)
fT (x2)
x2
x1
qfT(x1
) +(1−q)fT(x2
)
≥ fT (qx1
+(1−q)x2
)
fS (x2) fSfS (x1)
Figure 32 Illustration of the convex combination of two treatment
plans: treatment plans, defined via the fluence maps x1
and x2
,
correspond to points in the two-dimensional objective function space
spanned by the target and spinal cord objectives fT and fS. For convex
objective functions, an averaged plan is located between the linear
approximation (red line) and the true Pareto surface as indicated by the
green dot.
Intensity-Modulated Radiation Therapy Planning 463](https://image.slidesharecdn.com/2c9eb4f4-2888-4a25-884c-51d7148dc56c-151007162026-lva1-app6892/85/IMRT-33-320.jpg)




![that if the system calculates a dose that agrees with measure-
ments in the phantom, even though it is not the same as a dose
calculated for the patient in the same relative location in space,
then the system will most likely deliver the same dose in the
patient that it has calculated for the patient. Furthermore, if the
measured dose is within a tolerance at a couple of points, the
dose to the patient is likely to be within tolerance throughout
the treatment volume containing many thousands of beamlets.
Many other instruments containing large arrays of detectors
monitored by computer-based systems are available.
References
Ahamad A, Stevens CW, and Smythe WR (2003) Intensity-modulated radiation therapy:
A novel approach to the management of malignant pleural mesothelioma.
International Journal of Radiation Oncology, Biology, and Physics 55: 768–775.
Al-Mamgani A, Heemsbergen WD, Peeters ST, et al. (2008) Role of intensity-modulated
radiotherapy in reducing toxicity in dose escalation for localized prostate cancer.
International Journal of Radiation Oncology, Biology, Physics 73(3): 685–691.
Bertsekas DP (1999) Nonlinear Programming, 2nd ed. Belmont, MA: Athena Scientific.
Bokrantz R (2013) Multicriteria Optimization for Managing Tradeoffs in Radiation
Therapy Treatment Planning. PhD thesis, Department of Mathematics, KTH Royal
Institute of Technology, Stockholm, Sweden.
Bortfeld T (2006) IMRT: A review and preview. Physics in Medicine and Biology
51: R363–R379.
Bortfeld T, Beurkelbach J, Boesecke R, et al. (1990) Methods of image reconstruction
from projections applied to conformation radiotherapy. Physics in Medicine and
Biology 35: 1423–1434.
Bortfeld T, Boyer AL, Schlegel W, et al. (1994) Realization and verification of three
dimensional conformal radiotherapy with modulated fields. International Journal of
Radiation Oncology, Biology, Physics 30: 899–908.
Bortfeld T, Kahler DL, Waldron TJ, et al. (1994) X-ray field compensation with multileaf
collimators. International Journal of Radiation Oncology, Biology, Physics
28: 723–730.
Boyer AL, Biggs P, Galvin JM, et al. (2001) AAPM Report No. 72 Basic applications of
multileaf collimators. Report of Task Group 50. Madison, WI: Medical Physics
Publishing.
Boyer AL, Ezzell GA, and Yu CX (2012) Intensity-modulated radiation therapy.
In: Khan M and Gerbi BJ (eds.) Treatment Planning in Radiation Oncology, 3rd ed.,
pp. 201–228. Philadelphia, PA: Lippincott, Williams Wilkins.
Boyer AL and Li S (1997) Geometric analysis of light-field position of a multileaf
collimator with curved ends. Medical Physics 24: 757–762.
Brahme A (1988a) Optimization of stationary and moving beam radiation therapy
techniques. Radiotherapy and Oncology 12: 120–140.
Brahme A (1988b) Optimal setting of multileaf collimators in stationary beam radiation
therapy. Strahlentherapie 164: 343–350.
Brahme A, Ka¨llman P, and Lind B (1988) Absorbed dose and biological effect planning
in heavy ion therapy. VIIIth ICMP, San Antonio TX, Physics in Medicine and Biology
33(supplement 1): 73.
Brahme A, Roos J-E, and Lax I (1982) Solution of an integral equation encountered in
rotation therapy. Physics in Medicine and Biology 27: 1221–1229.
Carlsson F (2008) Combining segment generation with direct step-and-shoot
optimization in intensity-modulated radiation therapy. Medical Physics
35: 3828–3838.
Cassioli A and Unkelbach J (2013) Aperture shape optimization for IMRT treatment
planning. Physics in Medicine and Biology 58(2): 301–318.
Censor Y, Altschuler MD, and Powlis WD (1988) A computational solution of the
inverse problem in radiation-therapy treatment planning. Applied Mathematics and
Computation 25: 57–87.
Chui CS, Spirou S, and LoSasso T (1996) Testing of dynamic multileaf collimation.
Medical Physics 23: 635–641.
Clark VH, Chen Y, Wilkens J, Alaly JR, Zakaryan K, and Deasy JO (2008) IMRT
treatment planning for prostate cancer using prioritized prescription optimization
and mean-tail-dose functions. Linear Algebra and its Applications 428(5):
1345–1364.
Craft D, Halabi T, Shih HA, and Bortfeld TR (2006) Approximating convex Pareto
surfaces in multiobjective radiotherapy planning. Medical Physics 33(9):
3399–3407.
Dirkx MLP, Heijmen BJM, and Santvoort JPC (1998) Leaf trajectory calculation for
dynamic multileaf collimation to realize optimized fluence profiles. Physics in
Medicine and Biology 43: 1171–1184.
Ehrgott M, Gu¨ler C, Hamacher HW, and Shao L (2010) Mathematical optimization in
intensity-modulated radiation therapy. Annals of Operations Research
175: 309–365.
Ezzell GA, Burmeister JW, and Dogan N (2009) IMRT commissioning: Multiple
institution planning and dosimetry comparisons, a report from AAPM Task Group
119. Medical Physics 36: 5359–5373.
Ezzell GA, Galvin JM, Low D, et al. (2003) Guidance document on delivery, treatment
planning, and clinical implementation of IMRT: Report of the IMRT subcommittee of
the AAPM radiation therapy committee. Medical Physics 30: 2089–2115.
Forster KM, Smythe WR, Starkschall G, et al. (2003) Intensity-modulated radiotherapy
following extrapleural pneumonectomy for the treatment of malignant
mesothelioma: Clinical implementation. International Journal of Radiation
Oncology, Biology, and Physics 55: 606–616.
Hancock SL, Luxton G, Chen Y, et al. (2000) Intensity modulated radiotherapy for
localized or regional treatment of prostatic cancer. Clinical implementation and
improvement in acute tolerance [Abstract]. International Journal of Radiation
Oncology, Biology, Physics 48: 252–253.
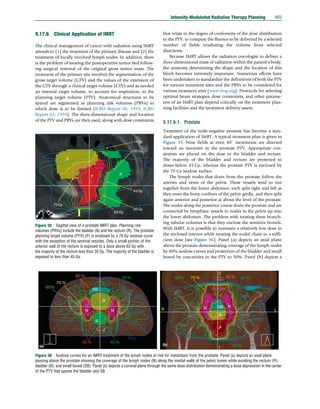
Figure 41 CT scan of a quasianatomical phantom overlaid with a computed IMRT dose distribution. The elliptical cylindrical phantom contains a
cylindrical insert that in turn contains a smaller cylindrical insert offset toward its periphery. An ionization chamber is inserted near the periphery of
the smaller tissue equivalent cylinder at point (I). This cylinder rotates within the larger cylinder whose circumference is made visible by air between
it and the rest of the phantom. Scales on the face of the phantom allow the user to rotate the two cylinders to angles that place the ionization chamber at
any x–y coordinate within the red circle whose diameter is 11.2 cm (e.g., the point (m1)). At (m1), the dose computed in the phantom is 204 cGy,
whereas the patient plan is computed using the MU that would deliver 200 cGy. If the quality assurance tolerance is 2%, a measurement between 200
and 208 cGy would allow the plan to be used for treatment. A second measurement is made at (m2) in a region being protected by a waist in the dose
distribution.
Intensity-Modulated Radiation Therapy Planning 469](https://image.slidesharecdn.com/2c9eb4f4-2888-4a25-884c-51d7148dc56c-151007162026-lva1-app6892/85/IMRT-39-320.jpg)
