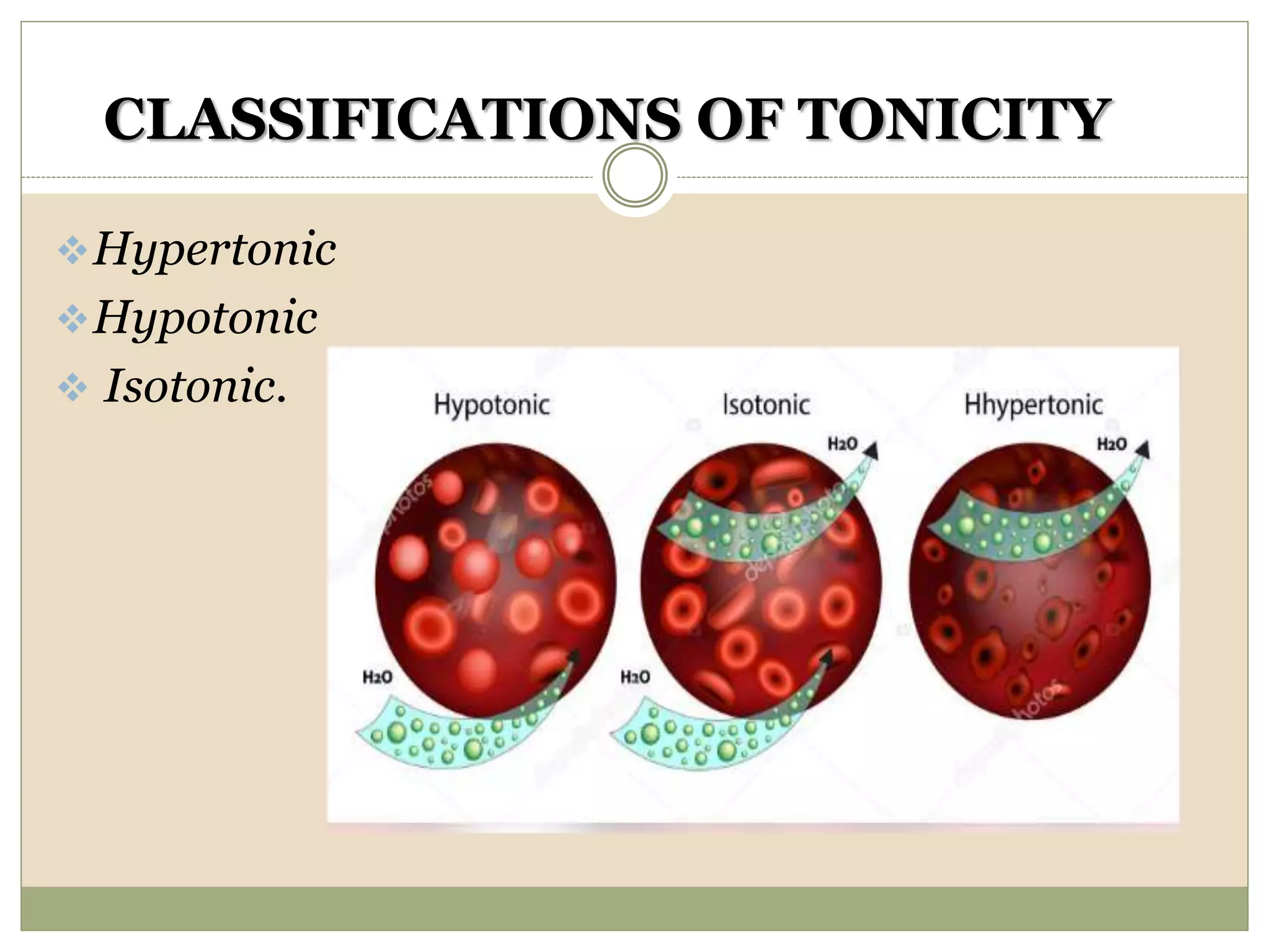
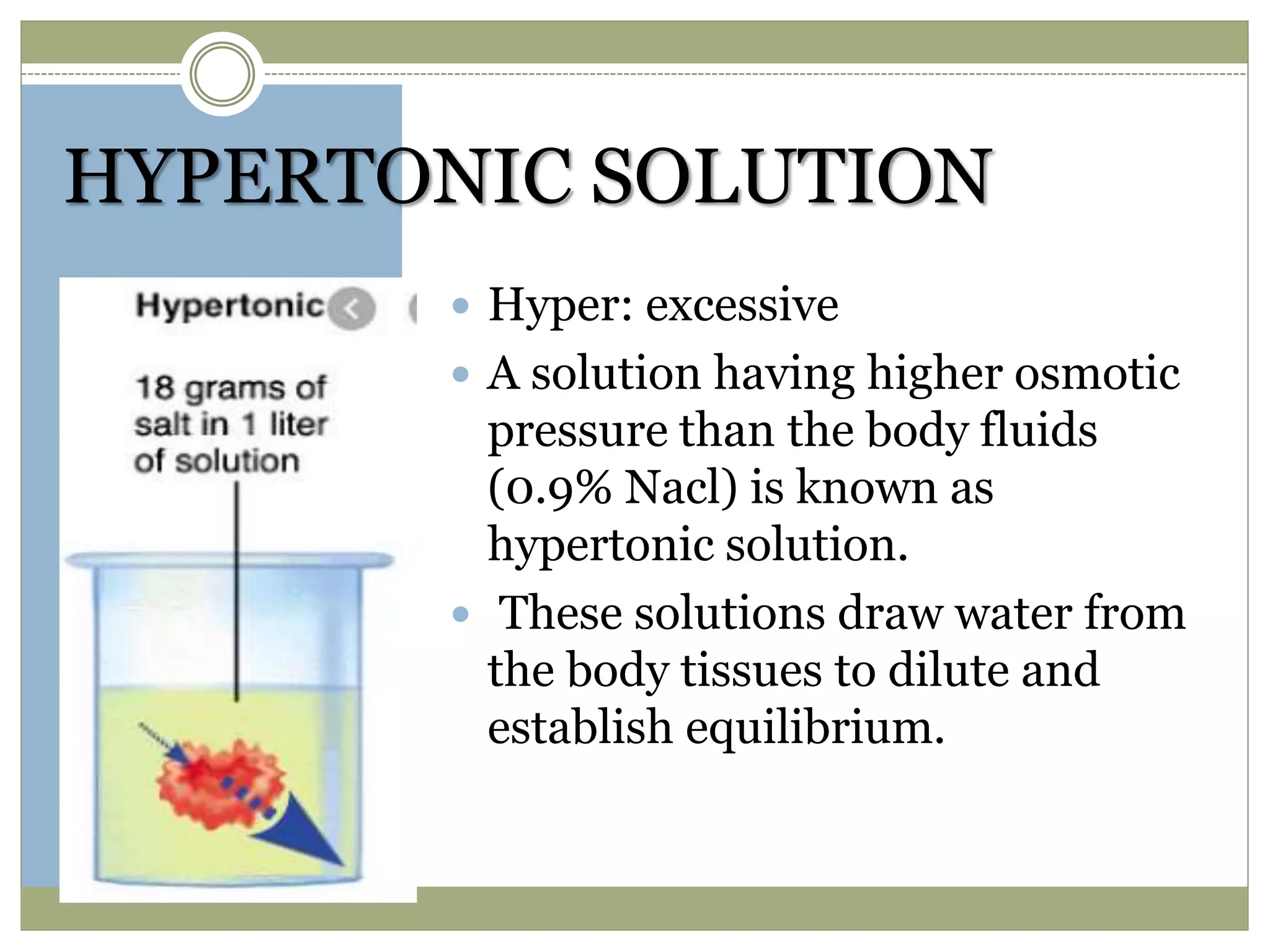
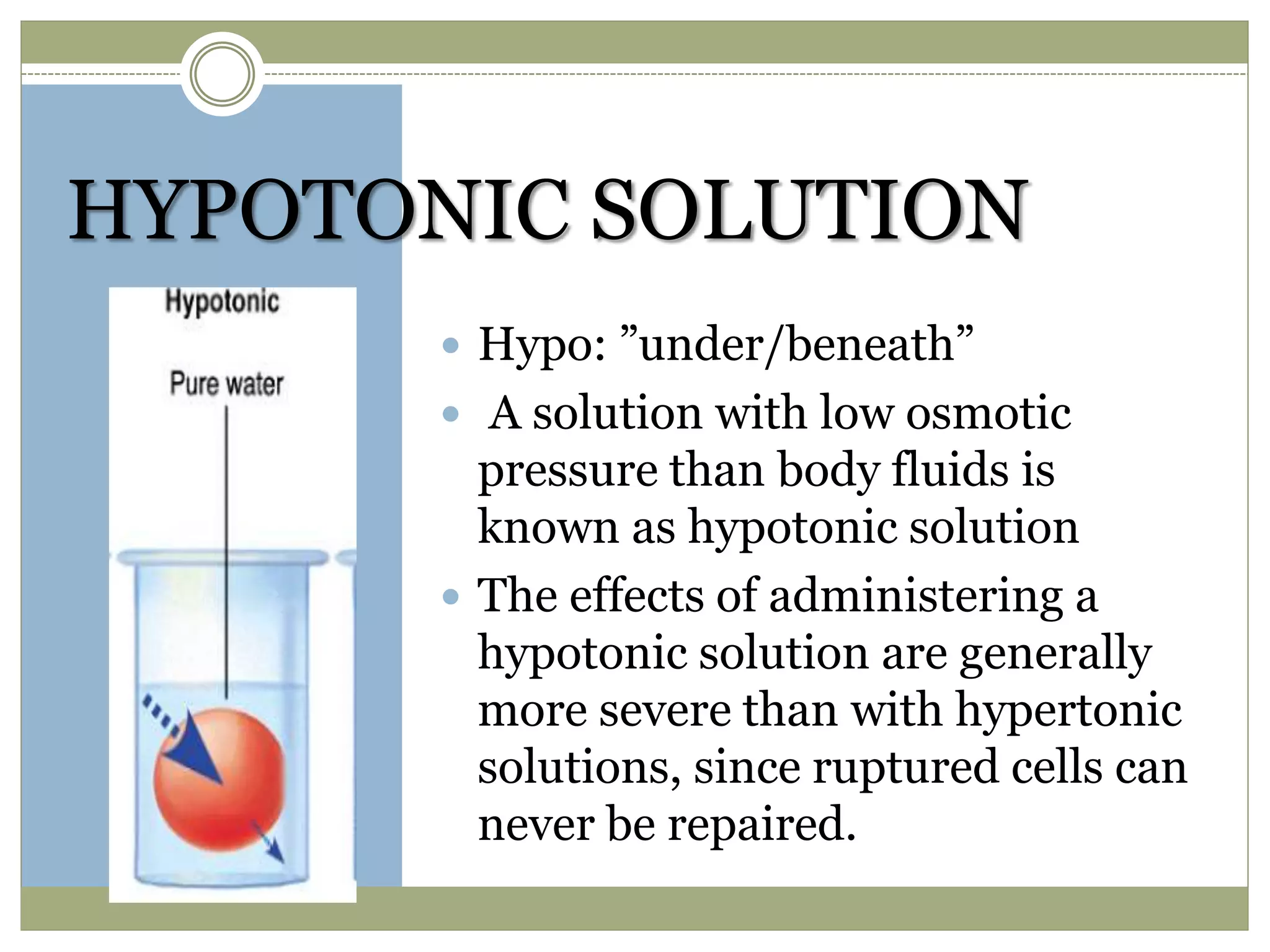
This document discusses the importance of tonicity and defines hypertonic, hypotonic, and isotonic solutions. It explains that tonicity is a measure of effective osmotic pressure between two solutions separated by a semi-permeable membrane. Hypertonic solutions have a higher concentration than body fluids and cause cells to shrink. Hypotonic solutions have a lower concentration than body fluids and cause cells to swell and burst. Isotonic solutions have the same concentration as body fluids and do not affect cell volume. The document provides examples of different concentration saline, dextrose, and lactated ringer's solutions and their classification as hypertonic, hypotonic or isotonic. It also discusses the importance of solutions being