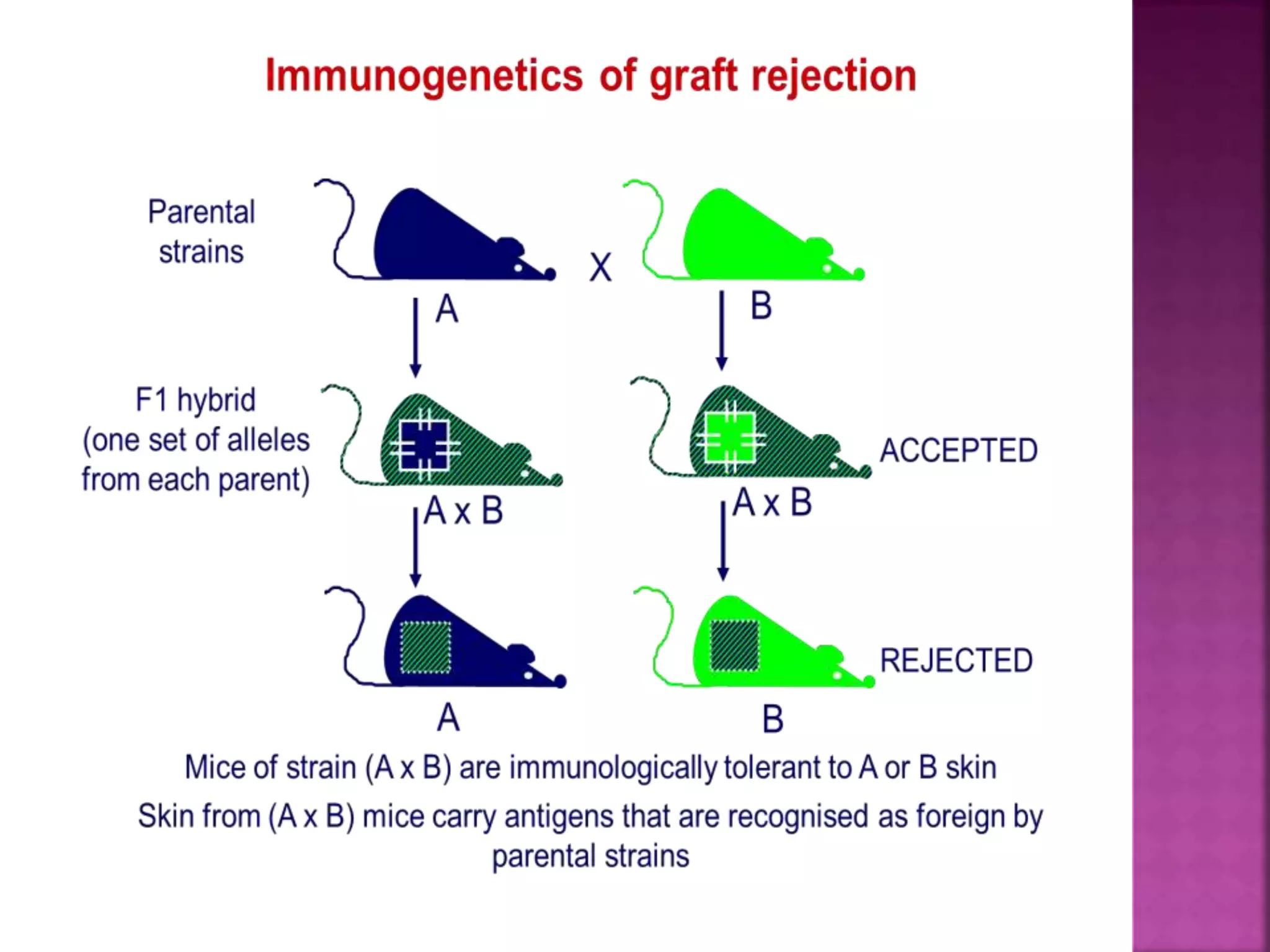
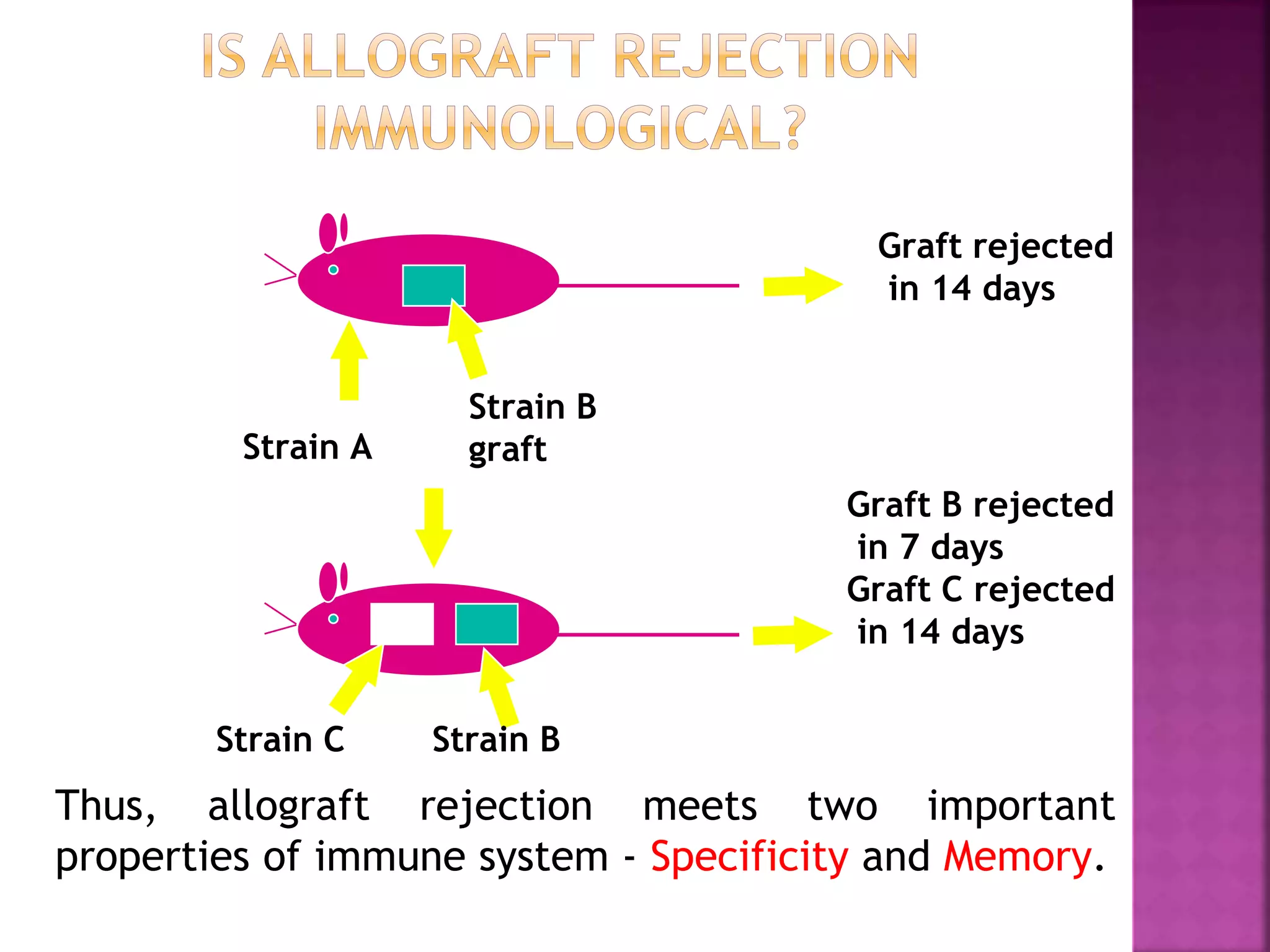
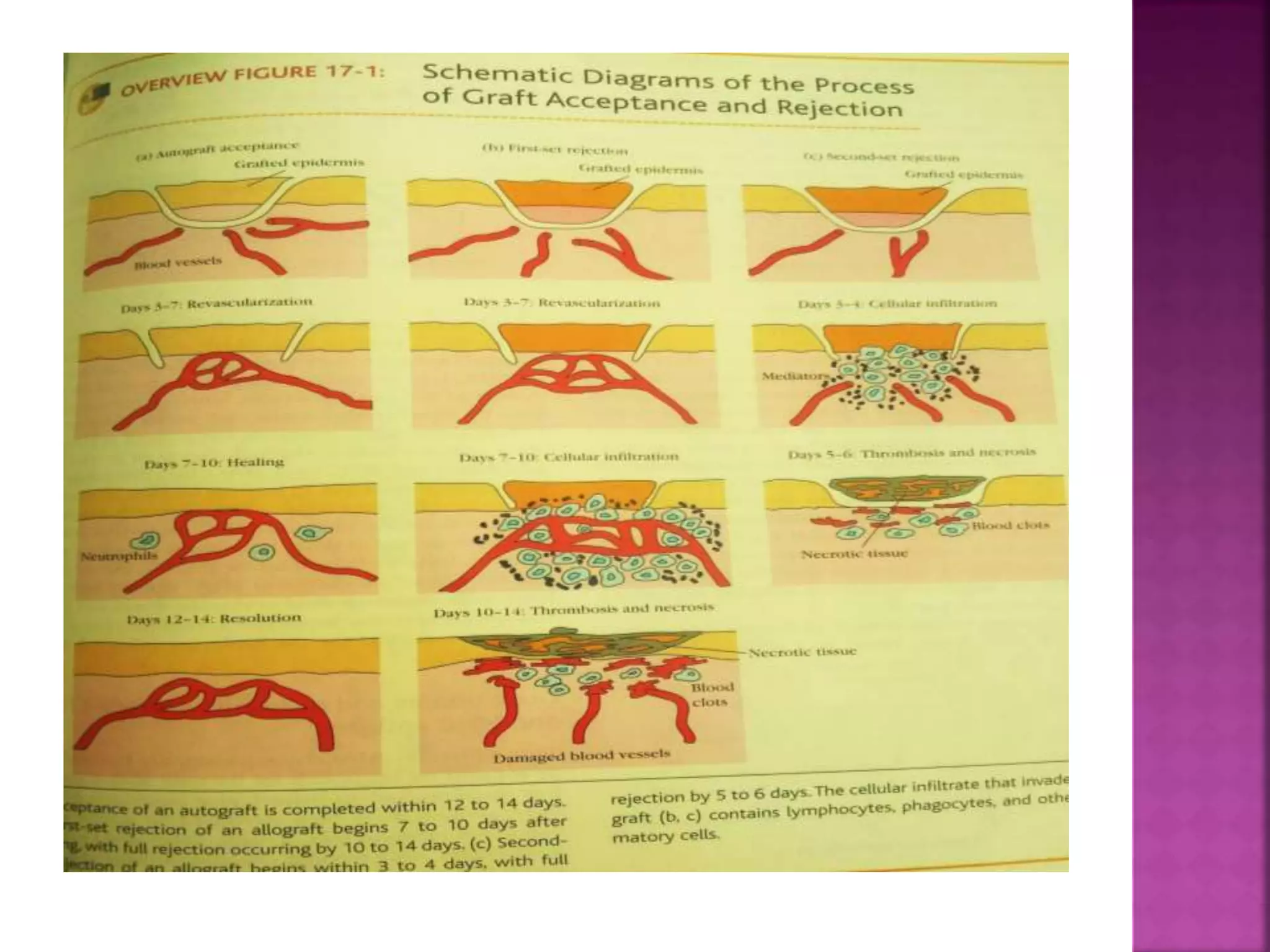
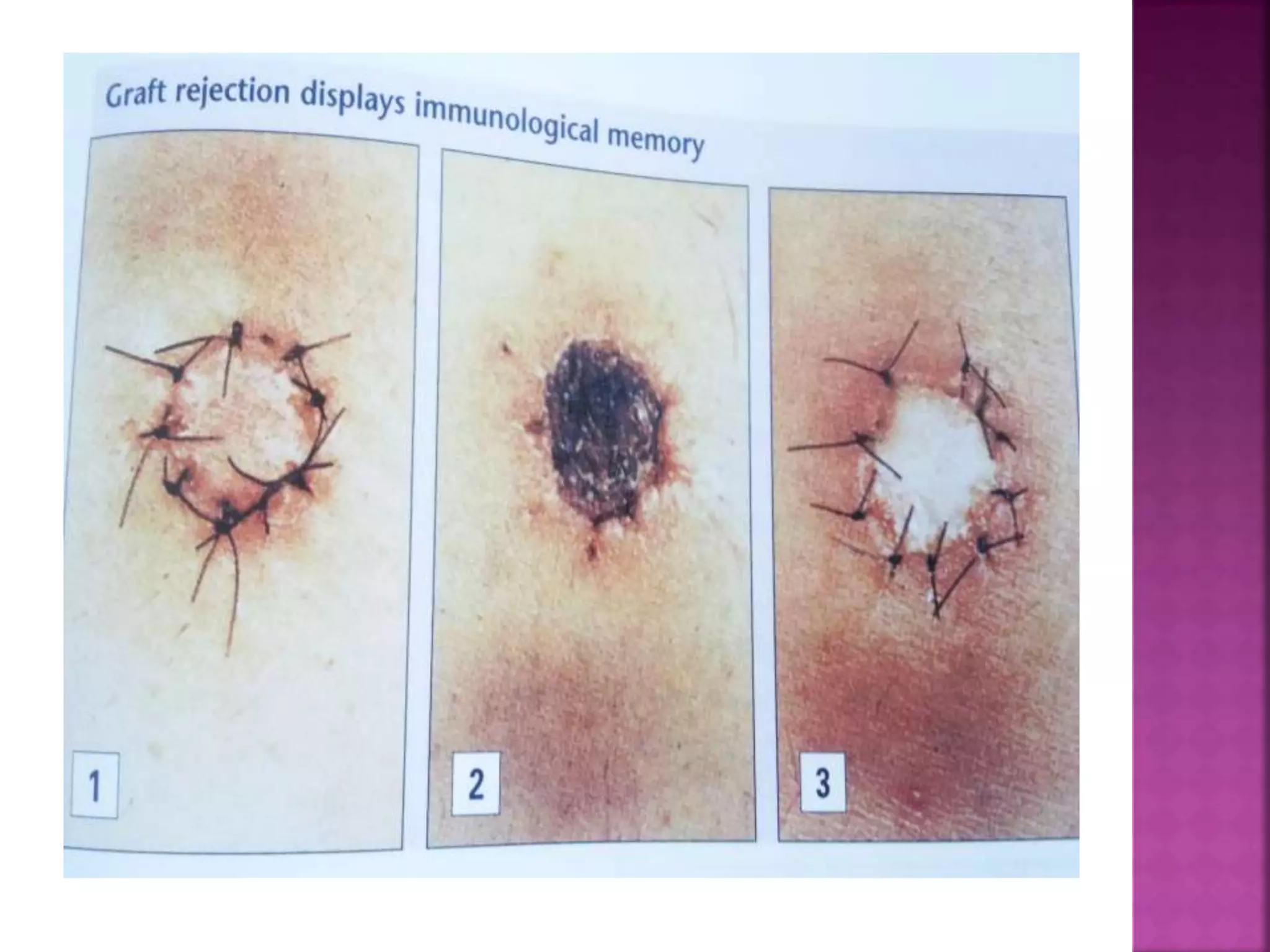
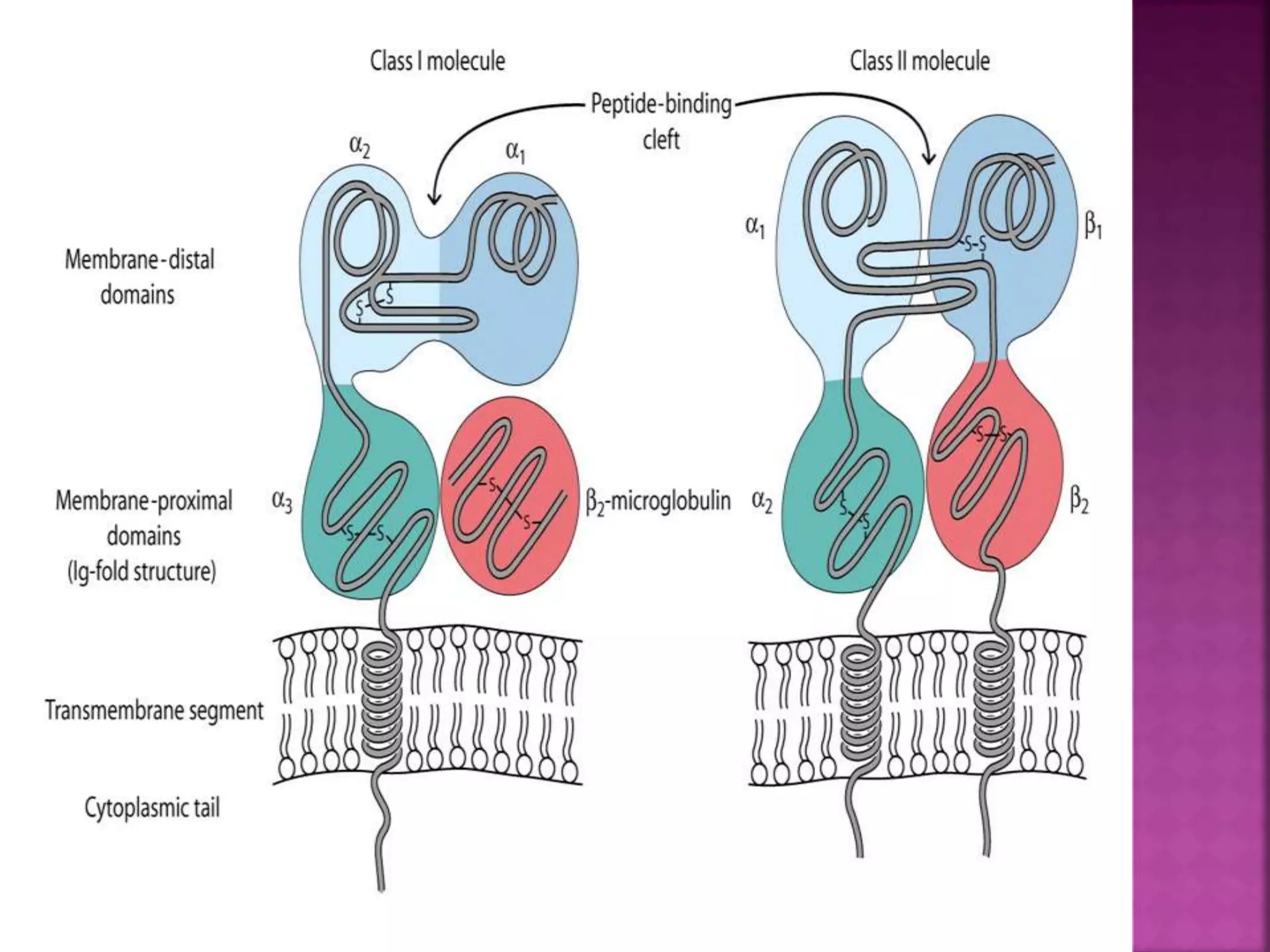
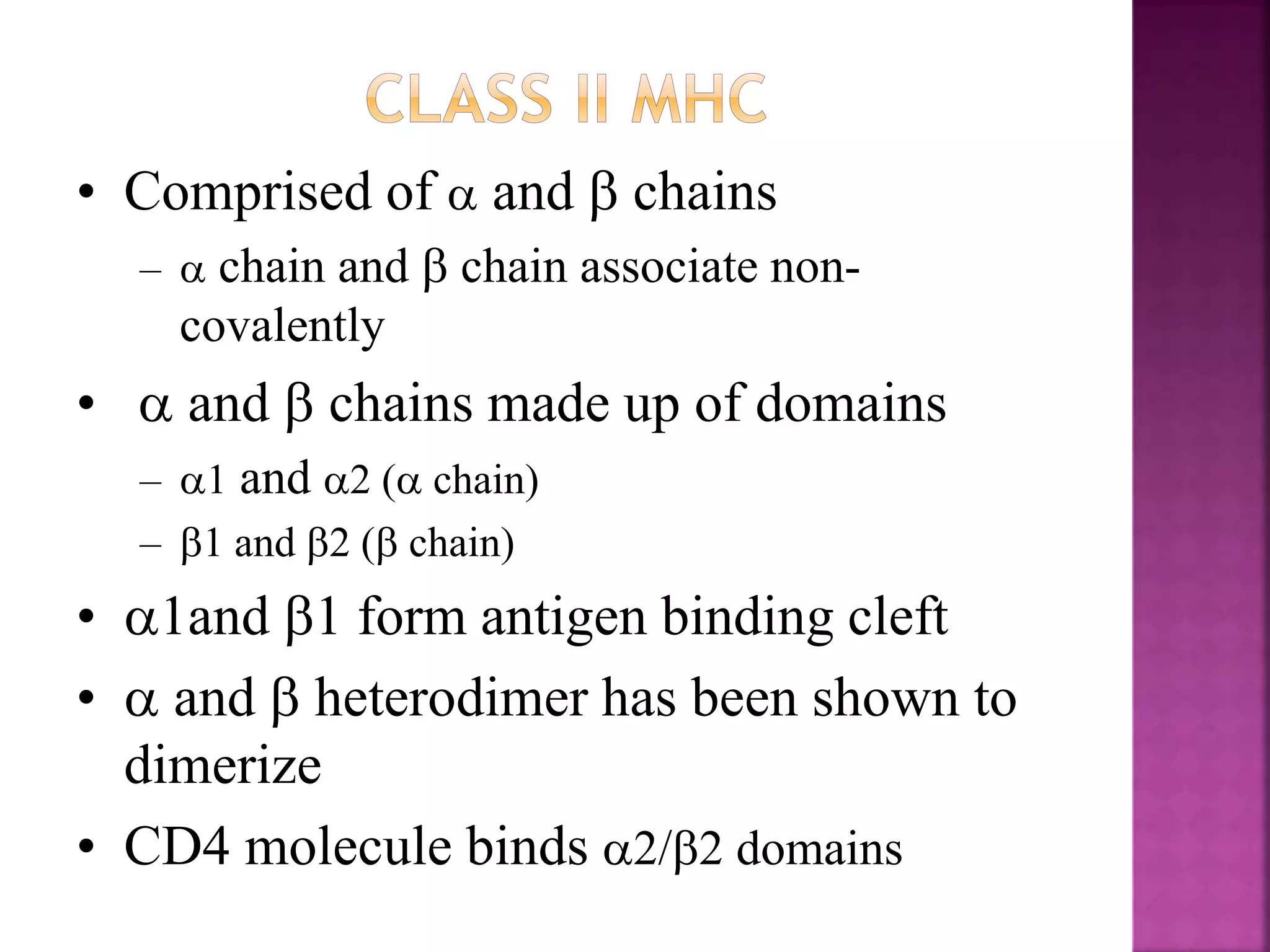
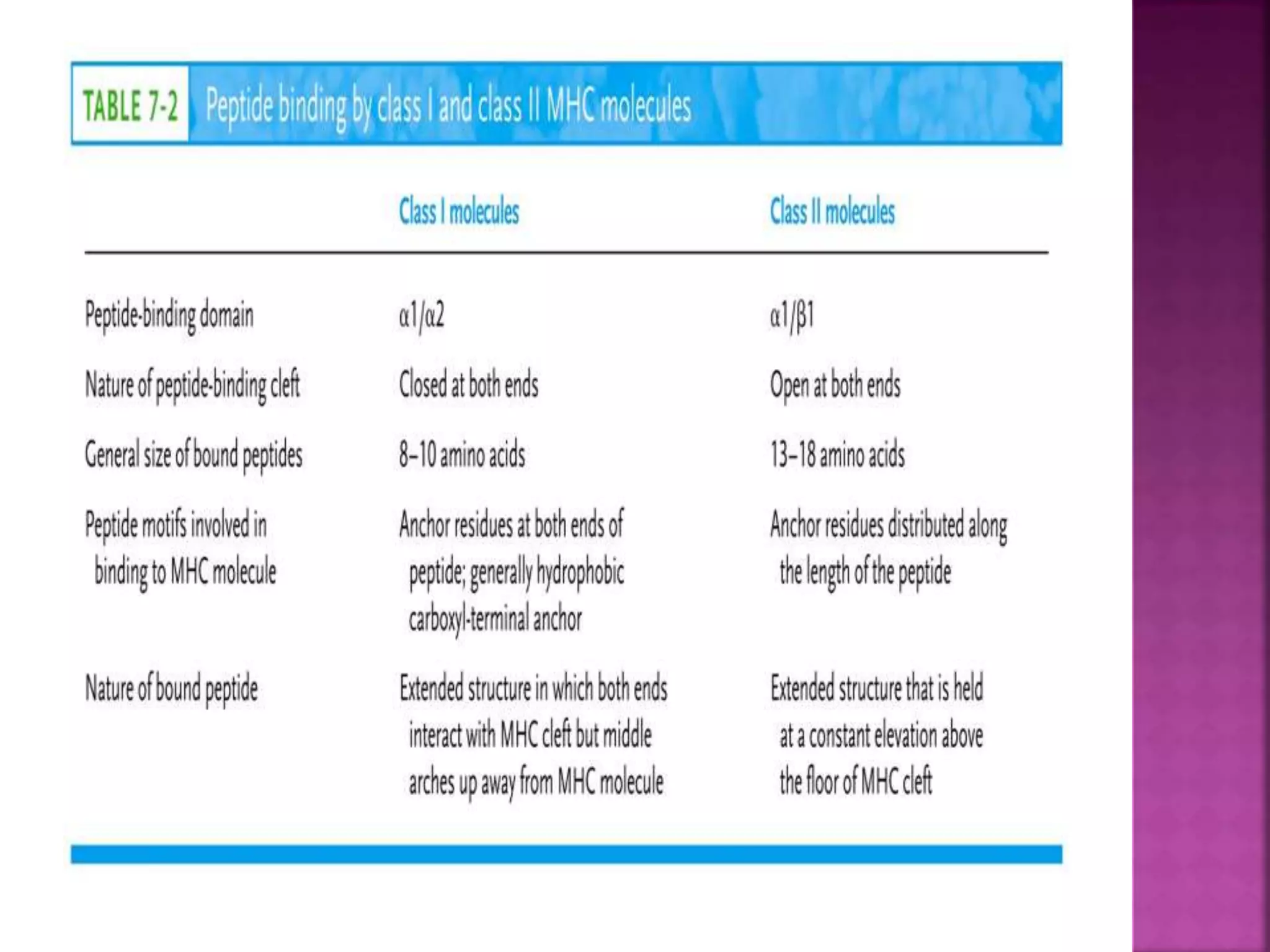
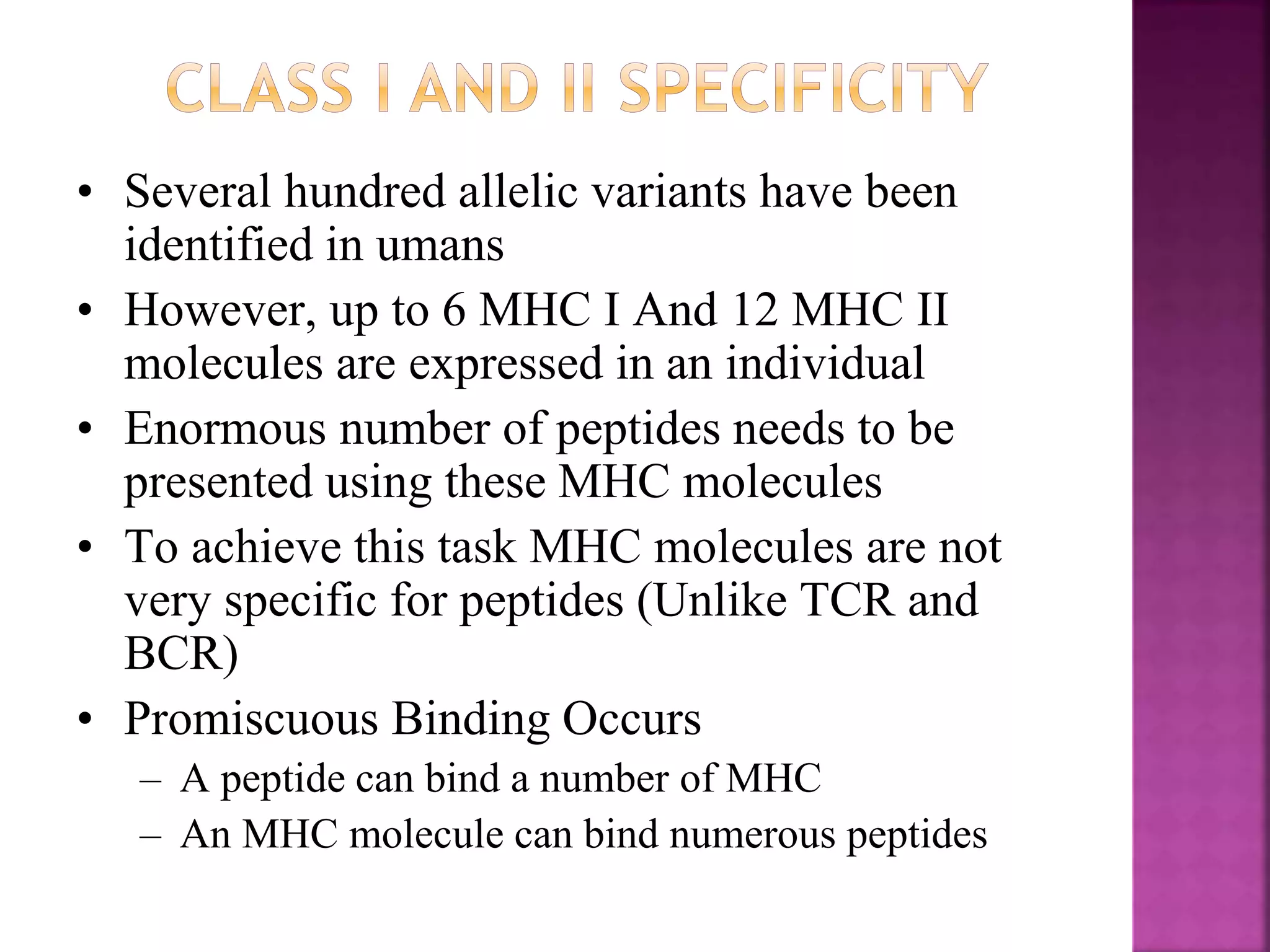
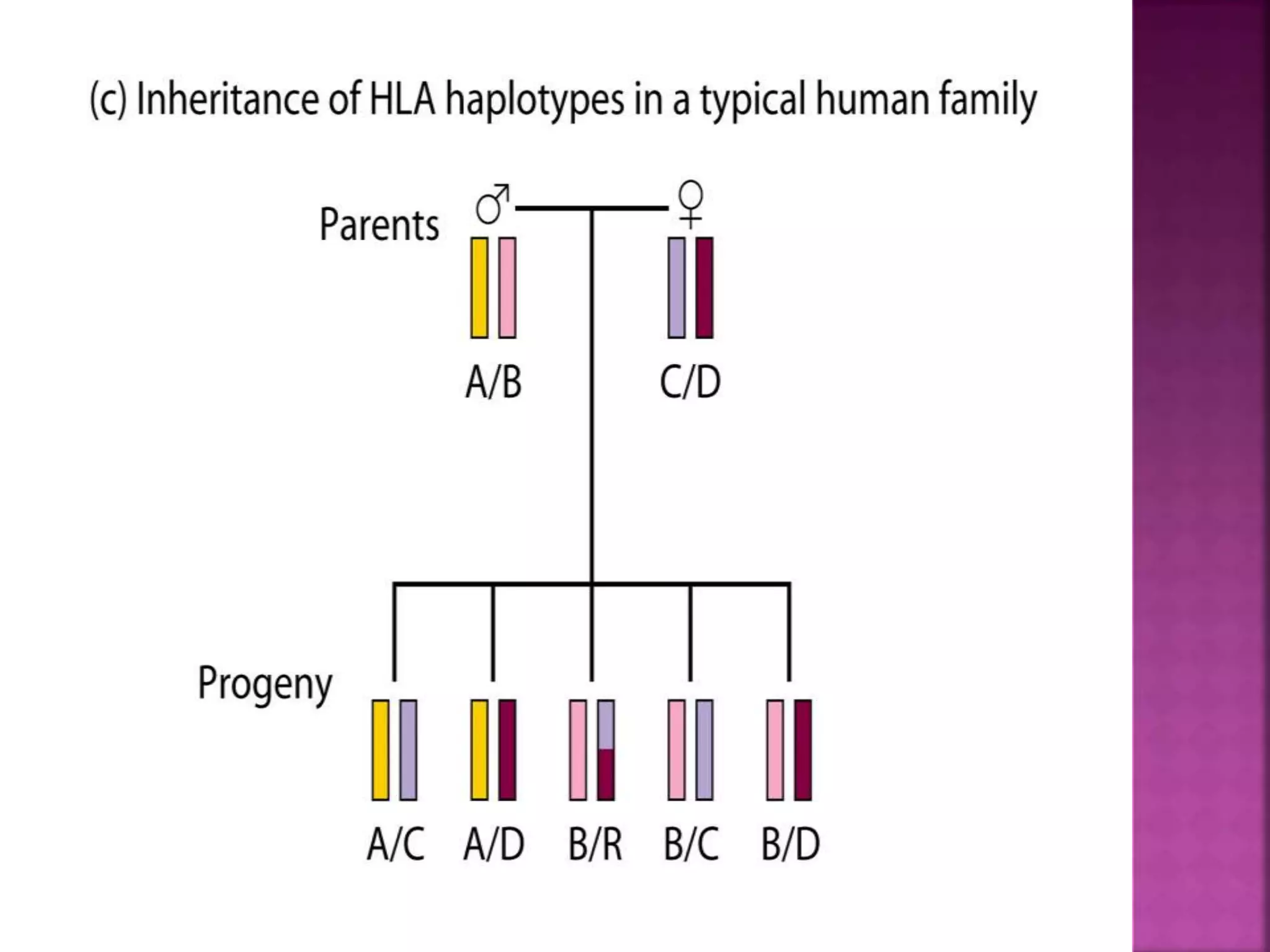
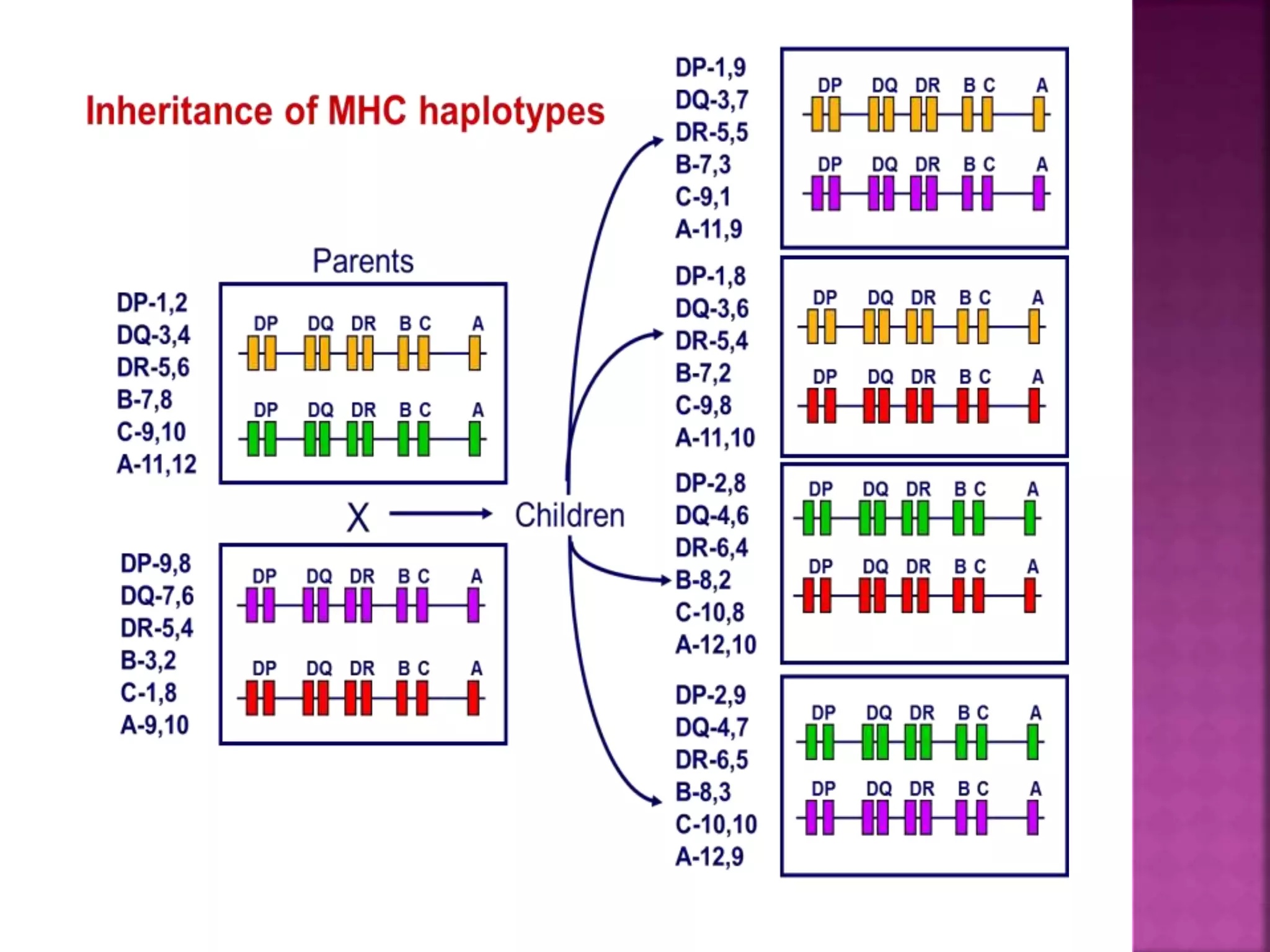
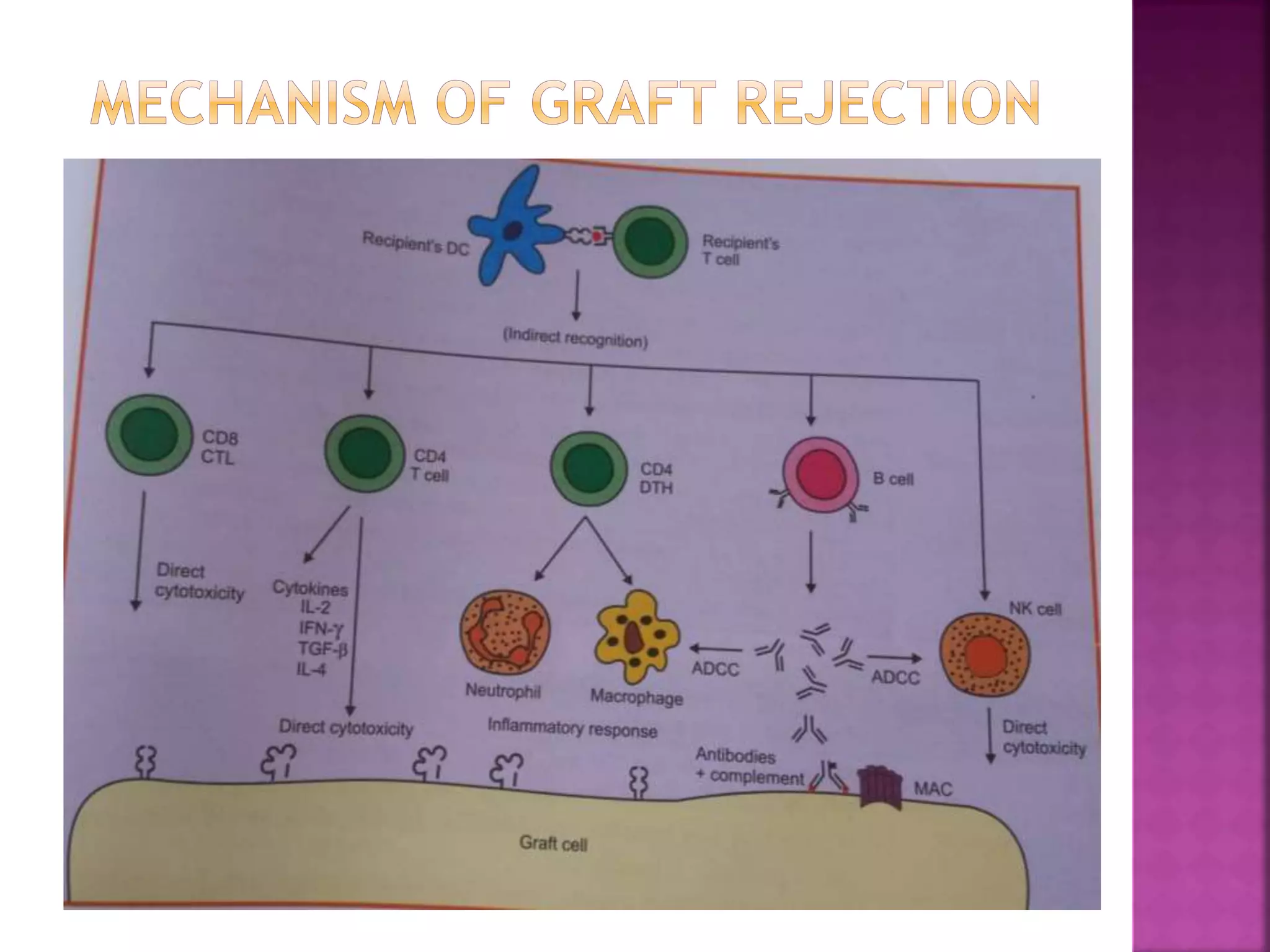
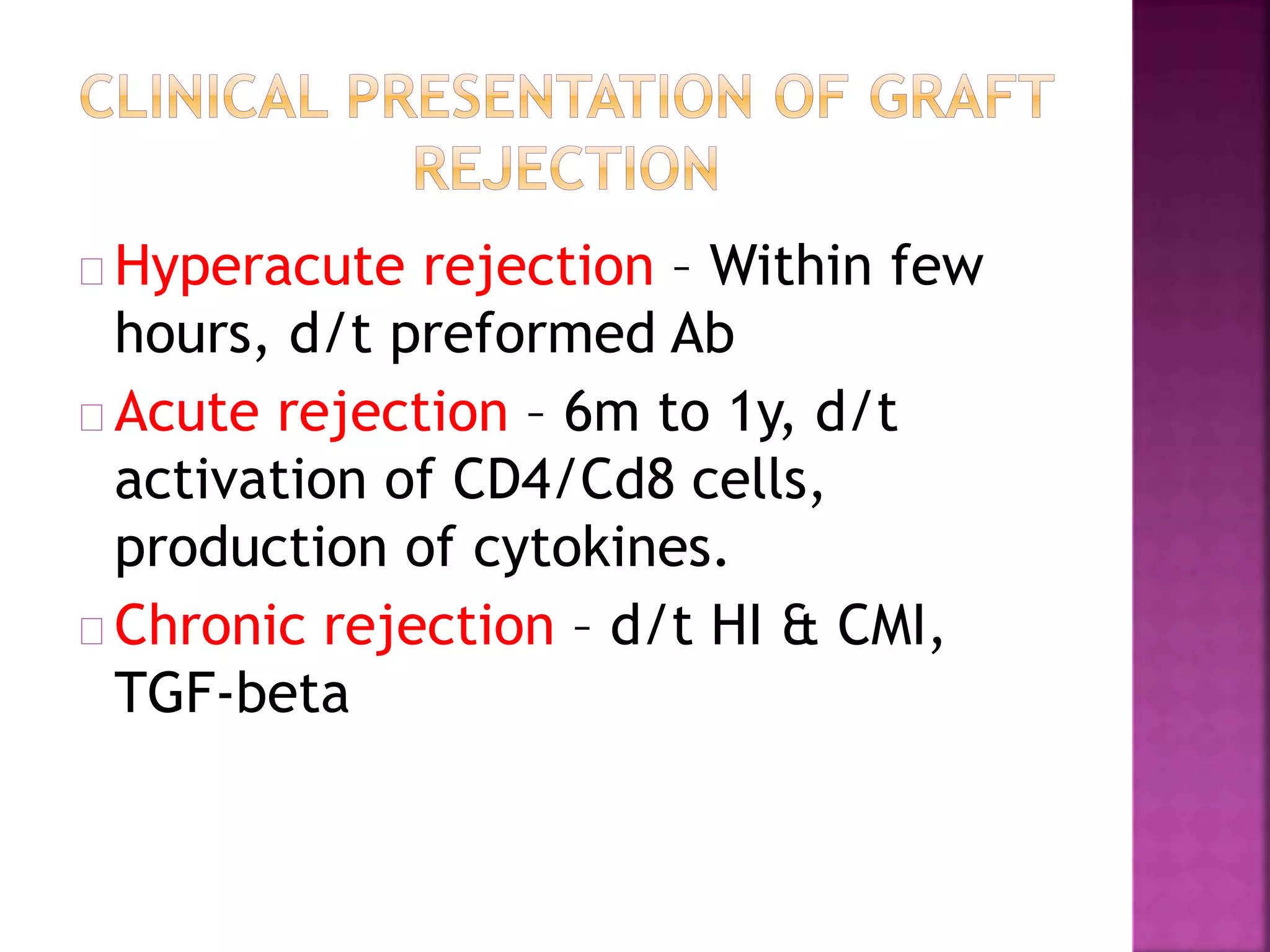
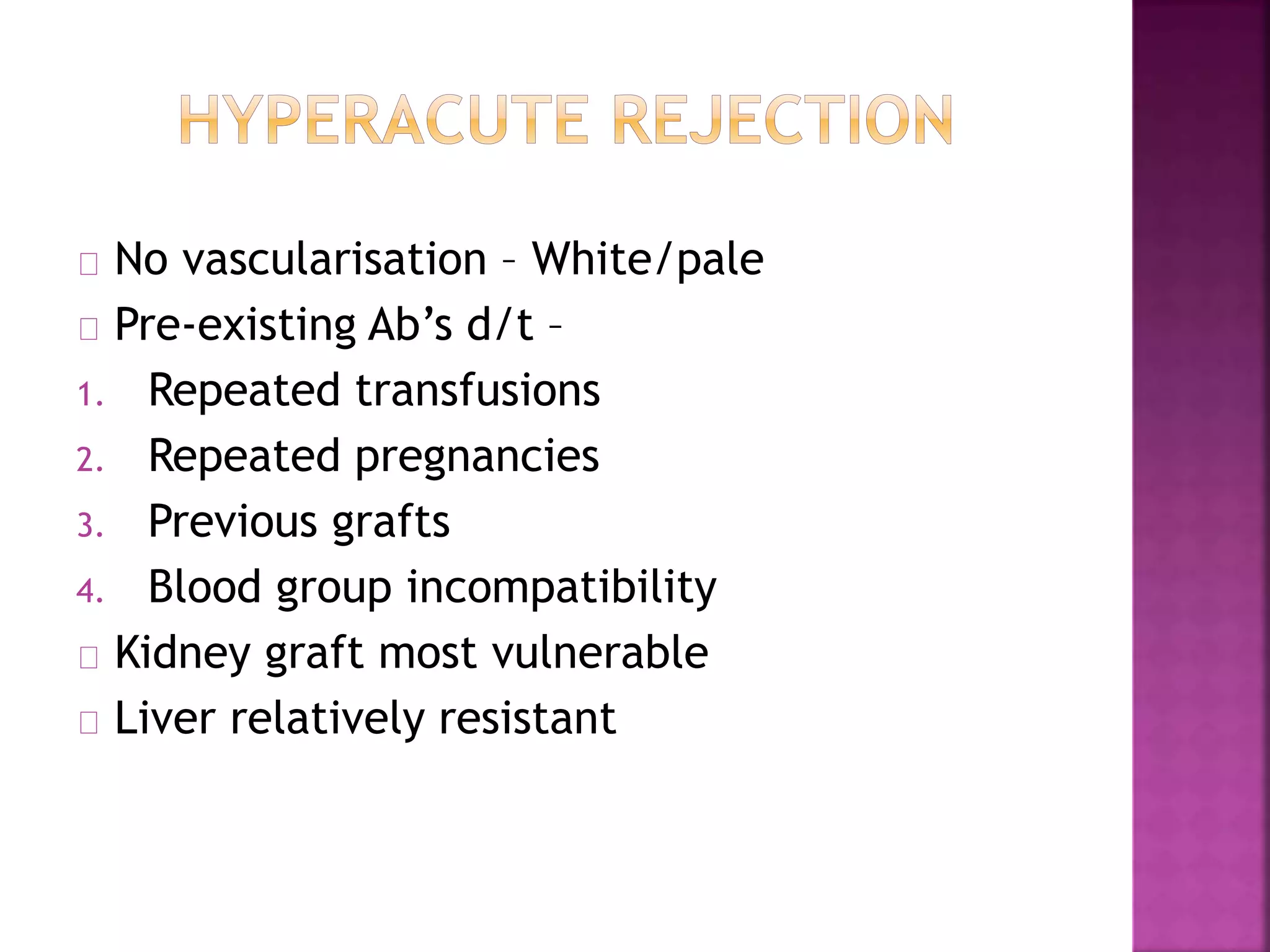
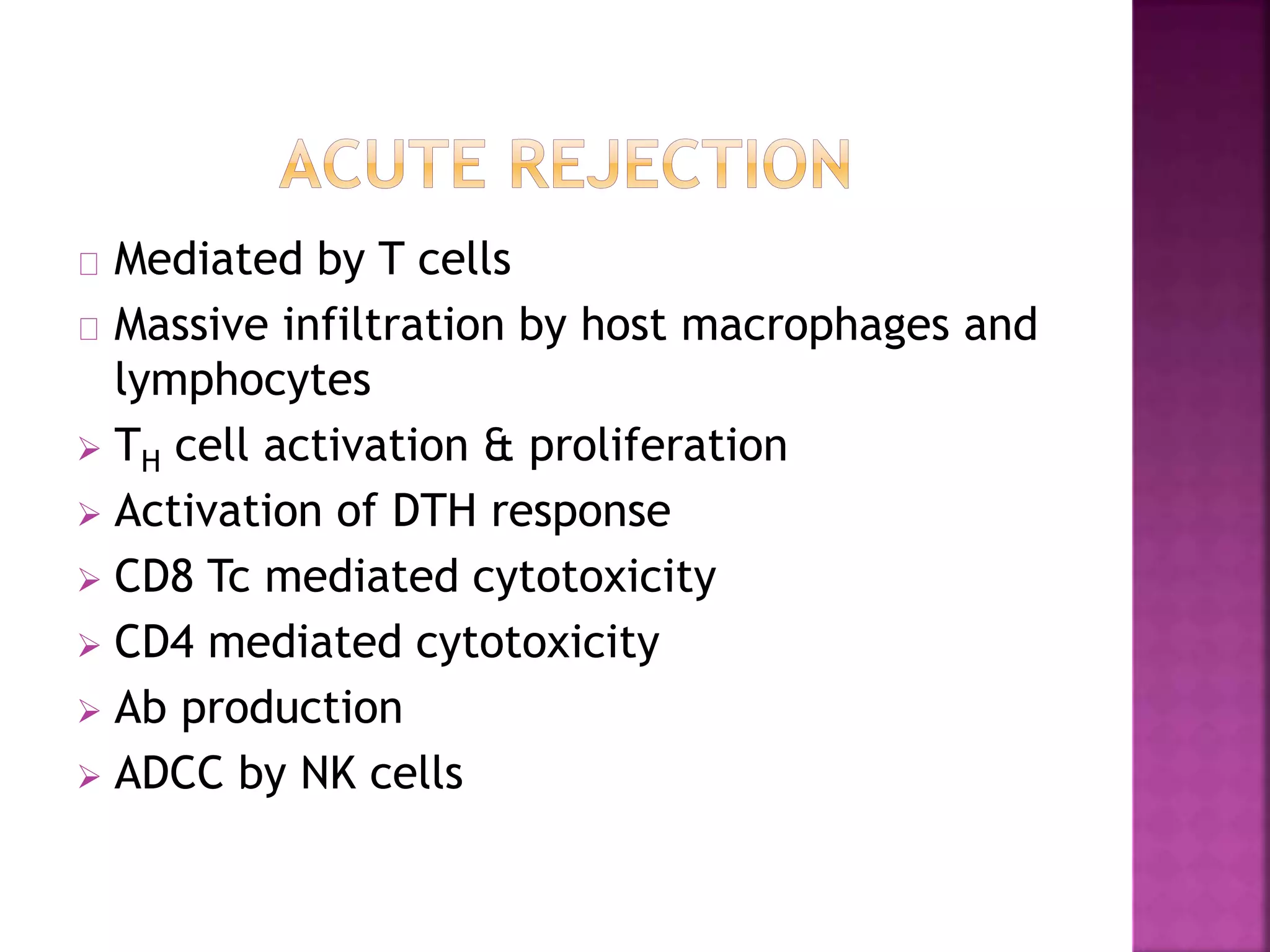
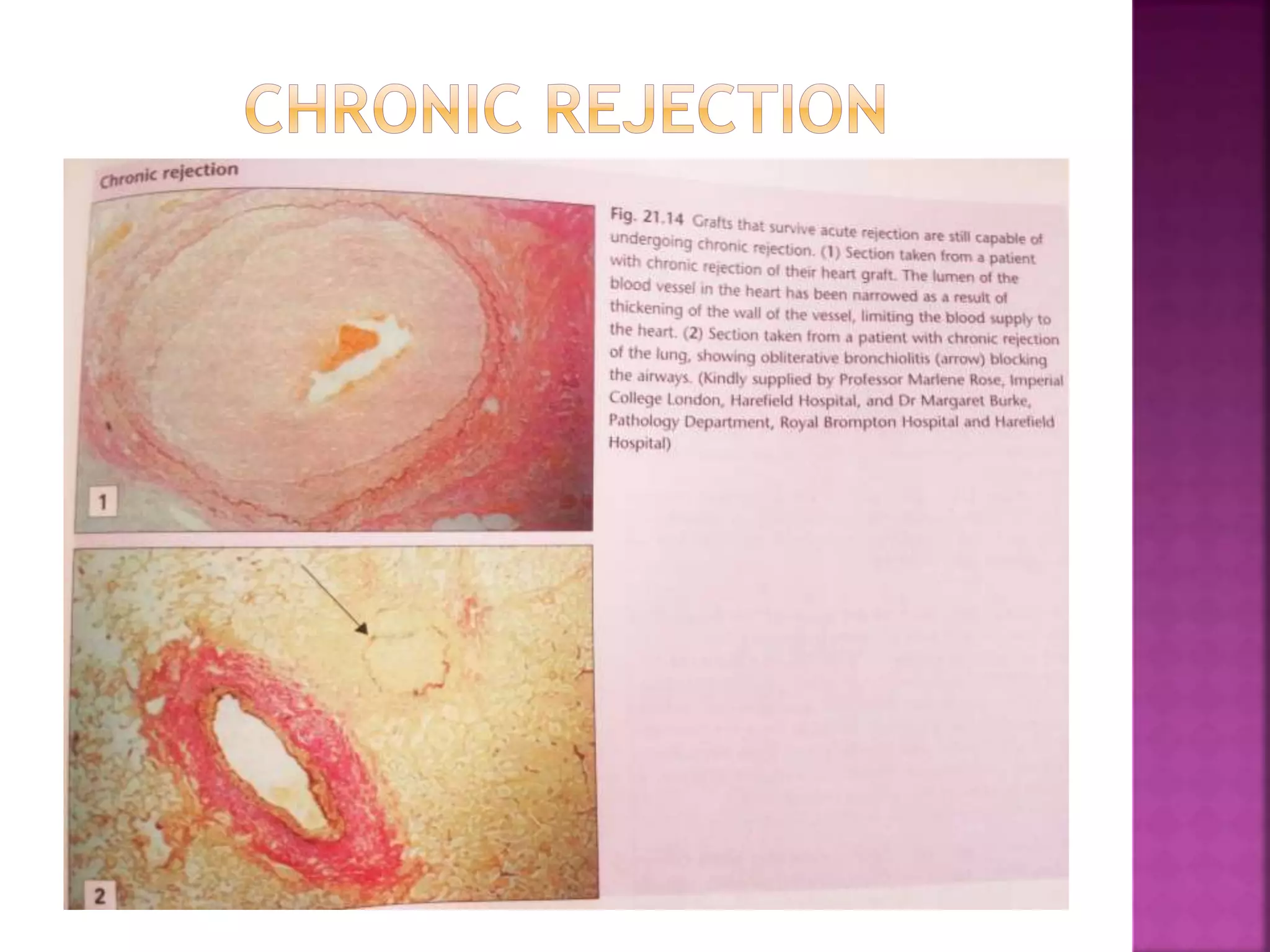
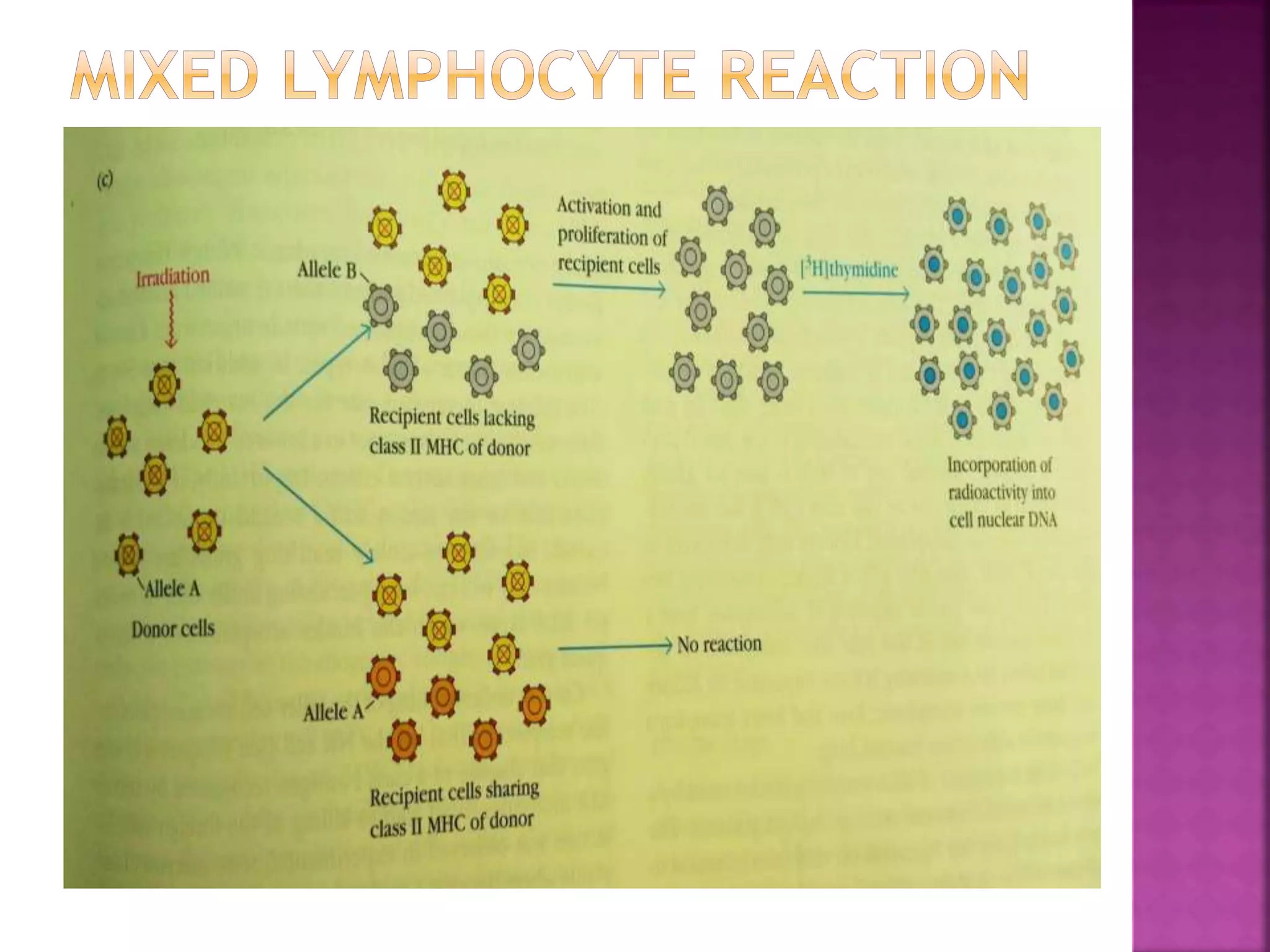
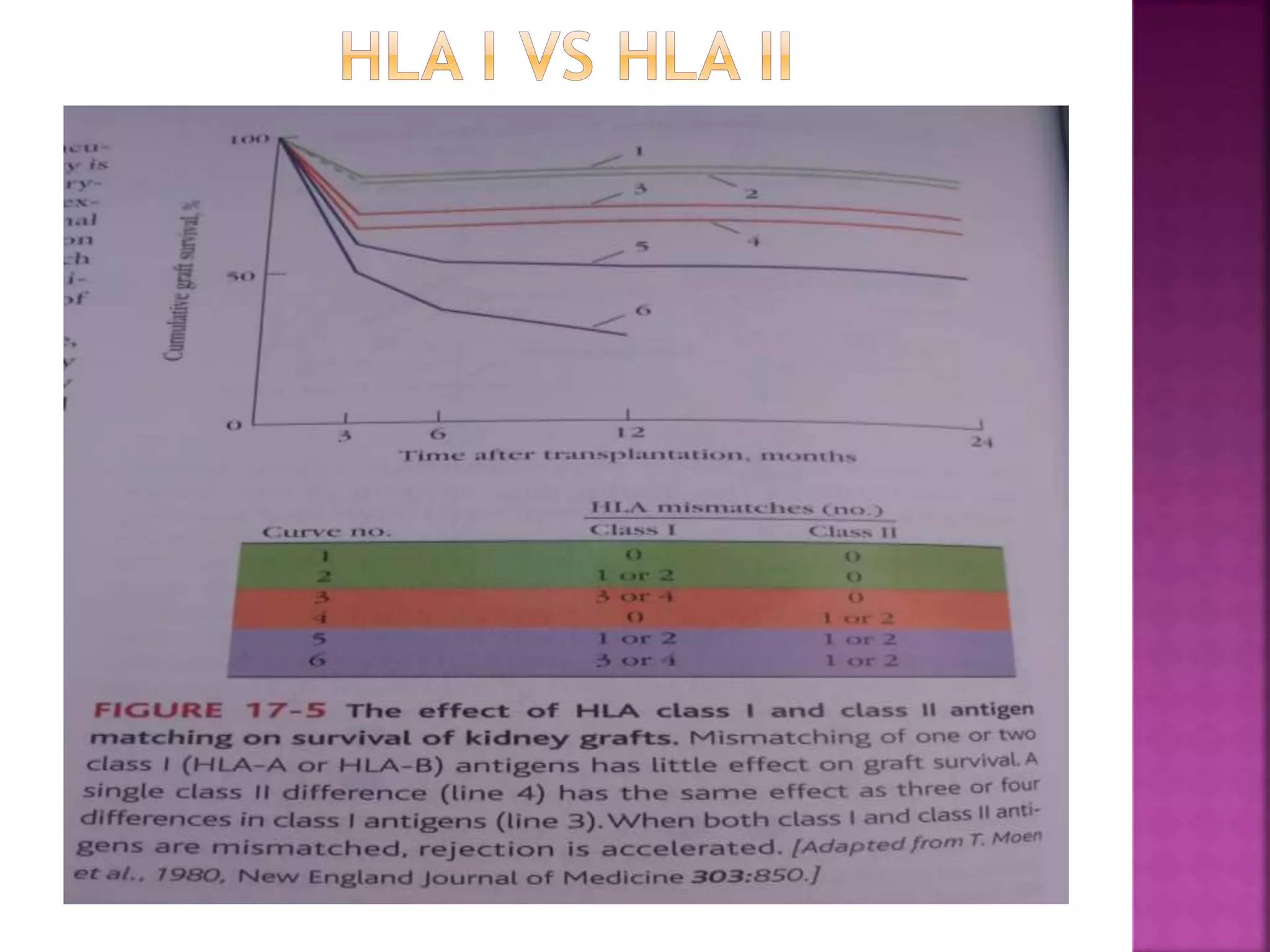
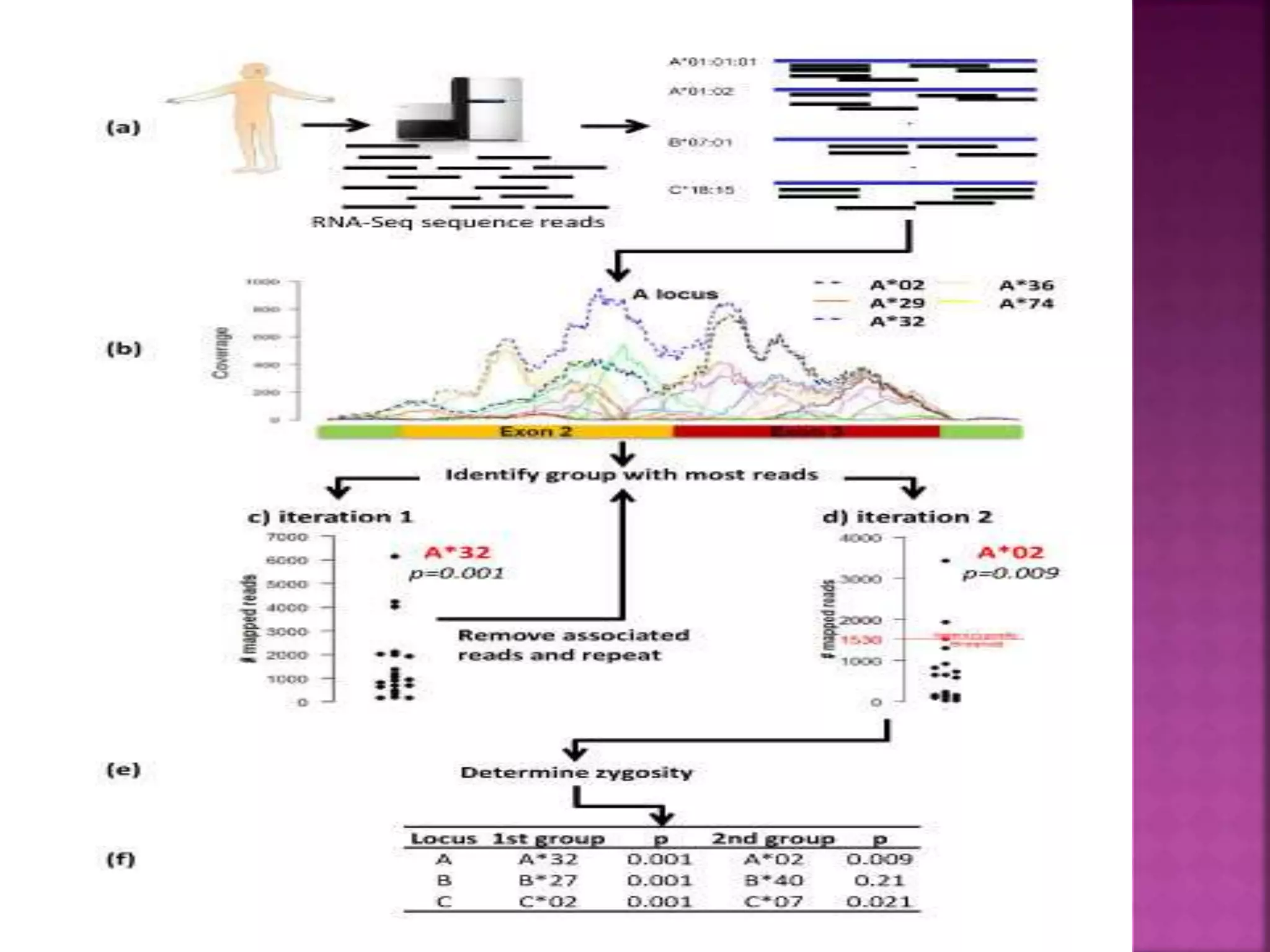
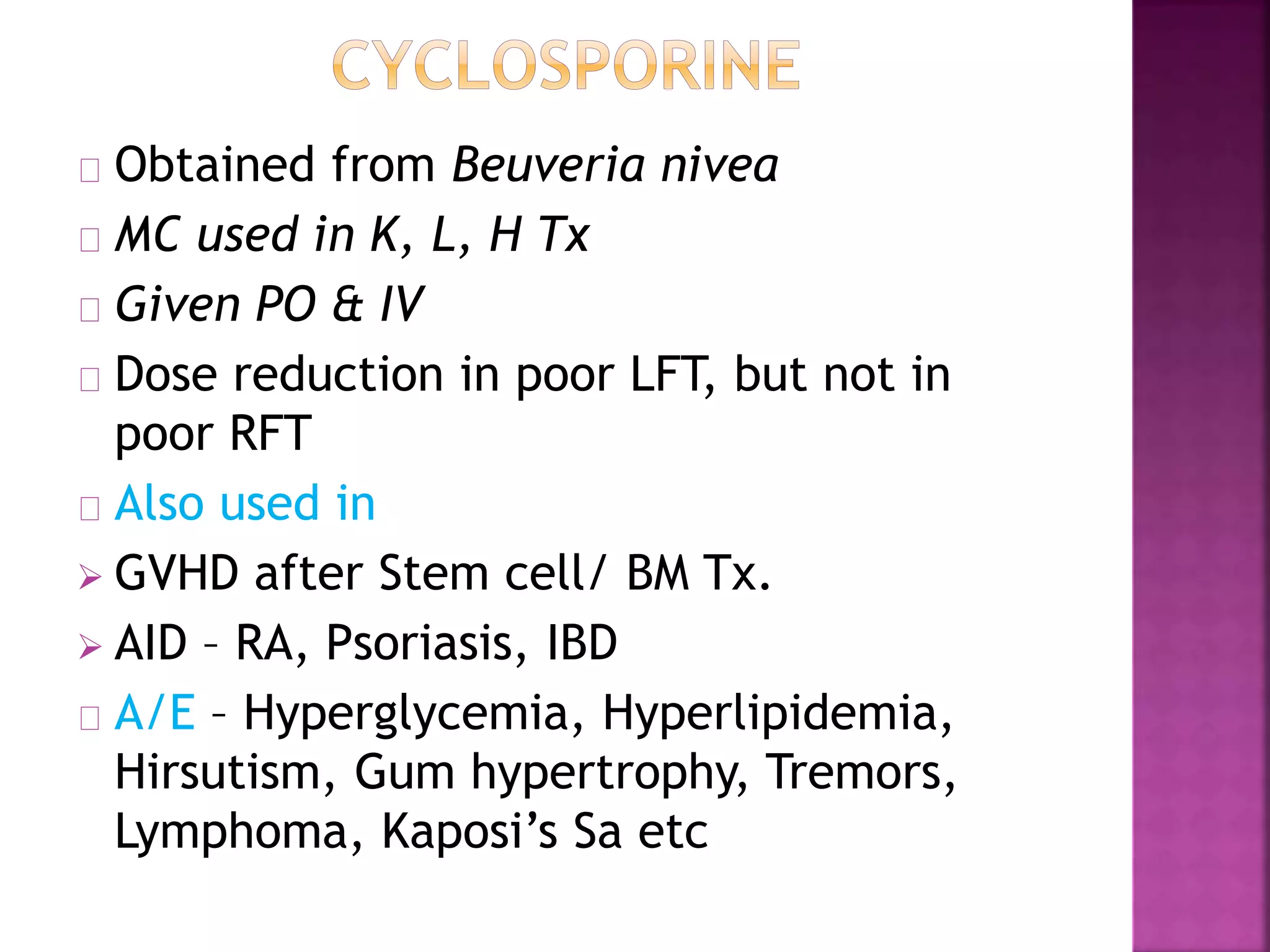
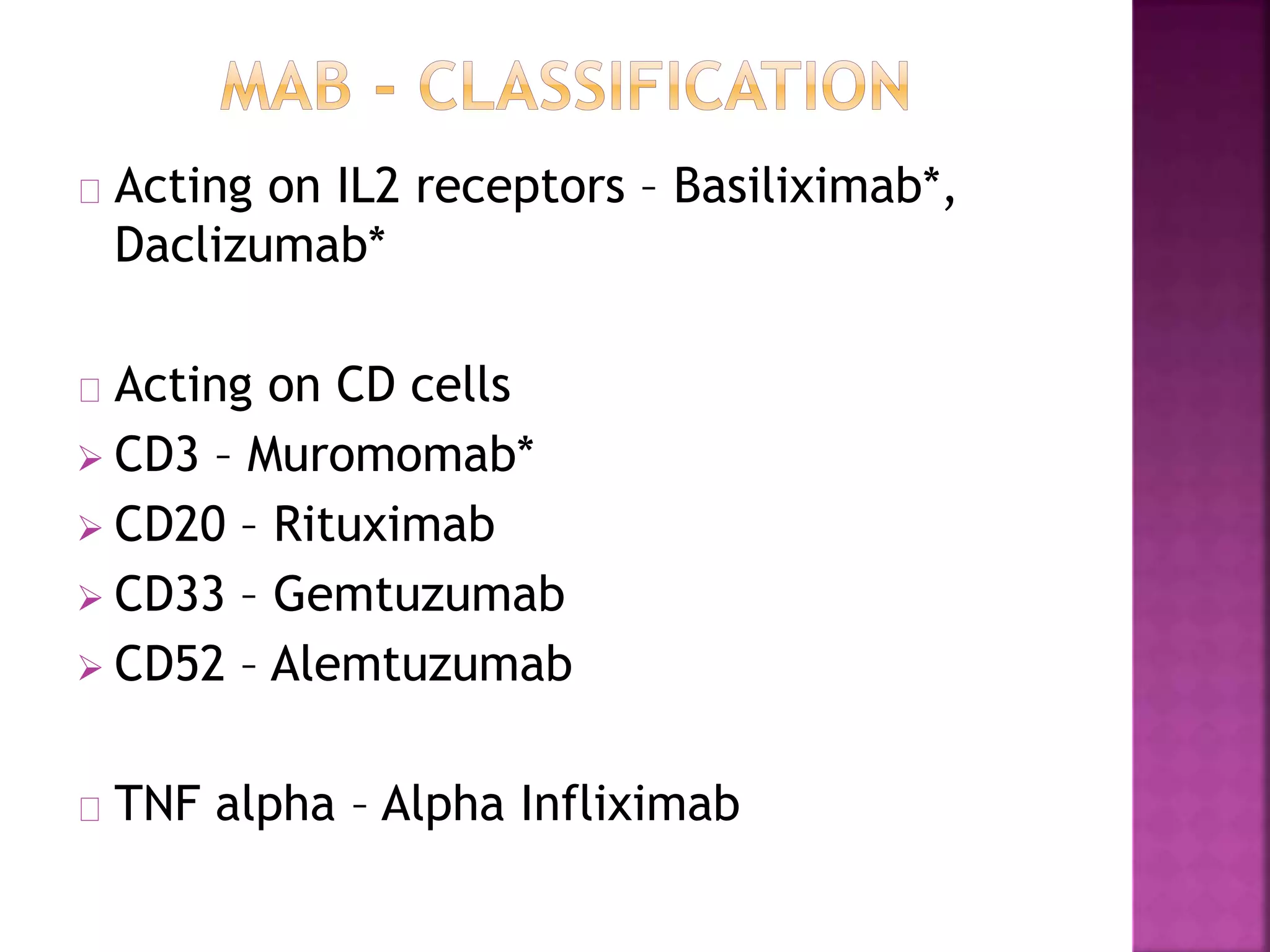
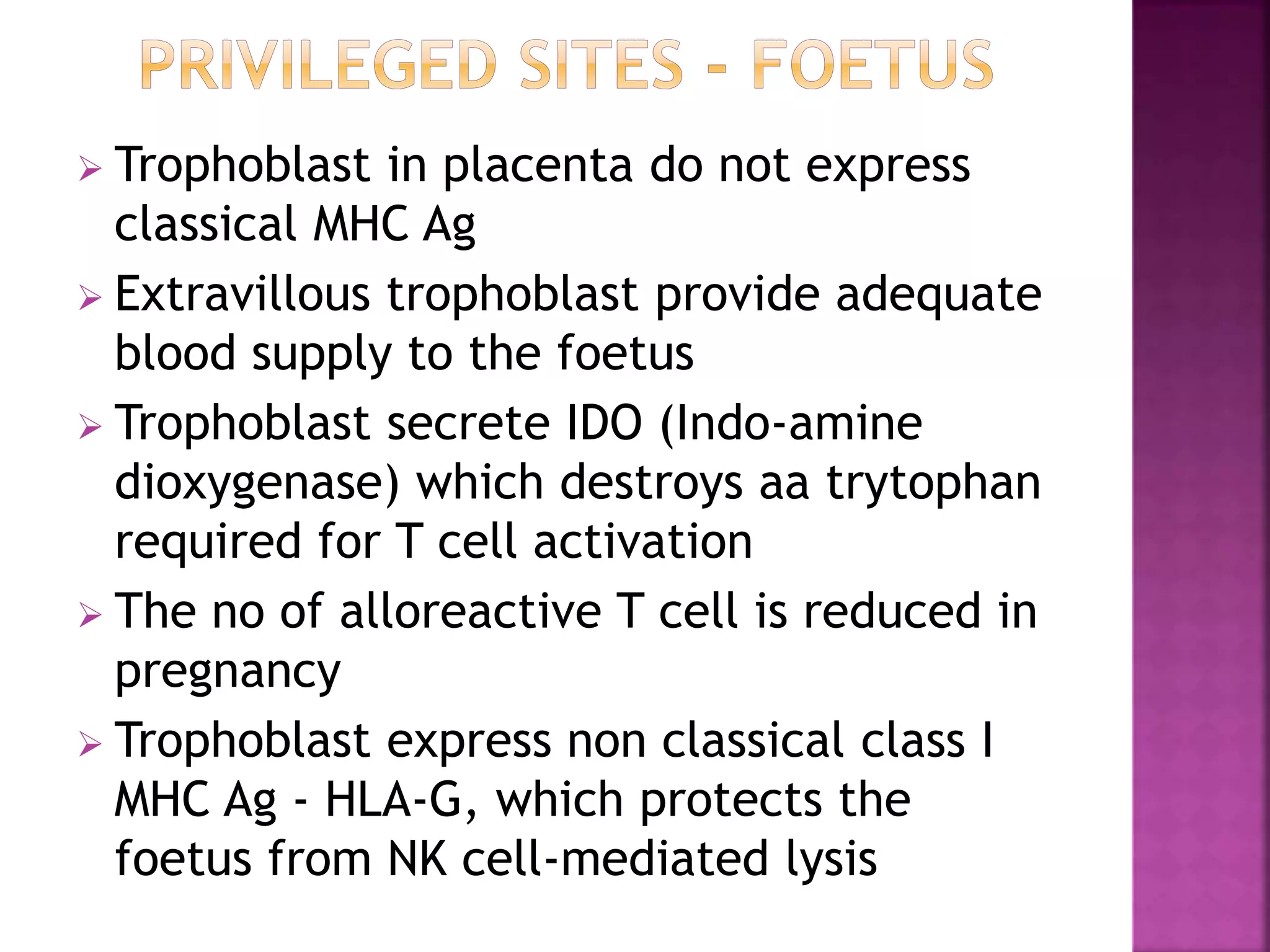
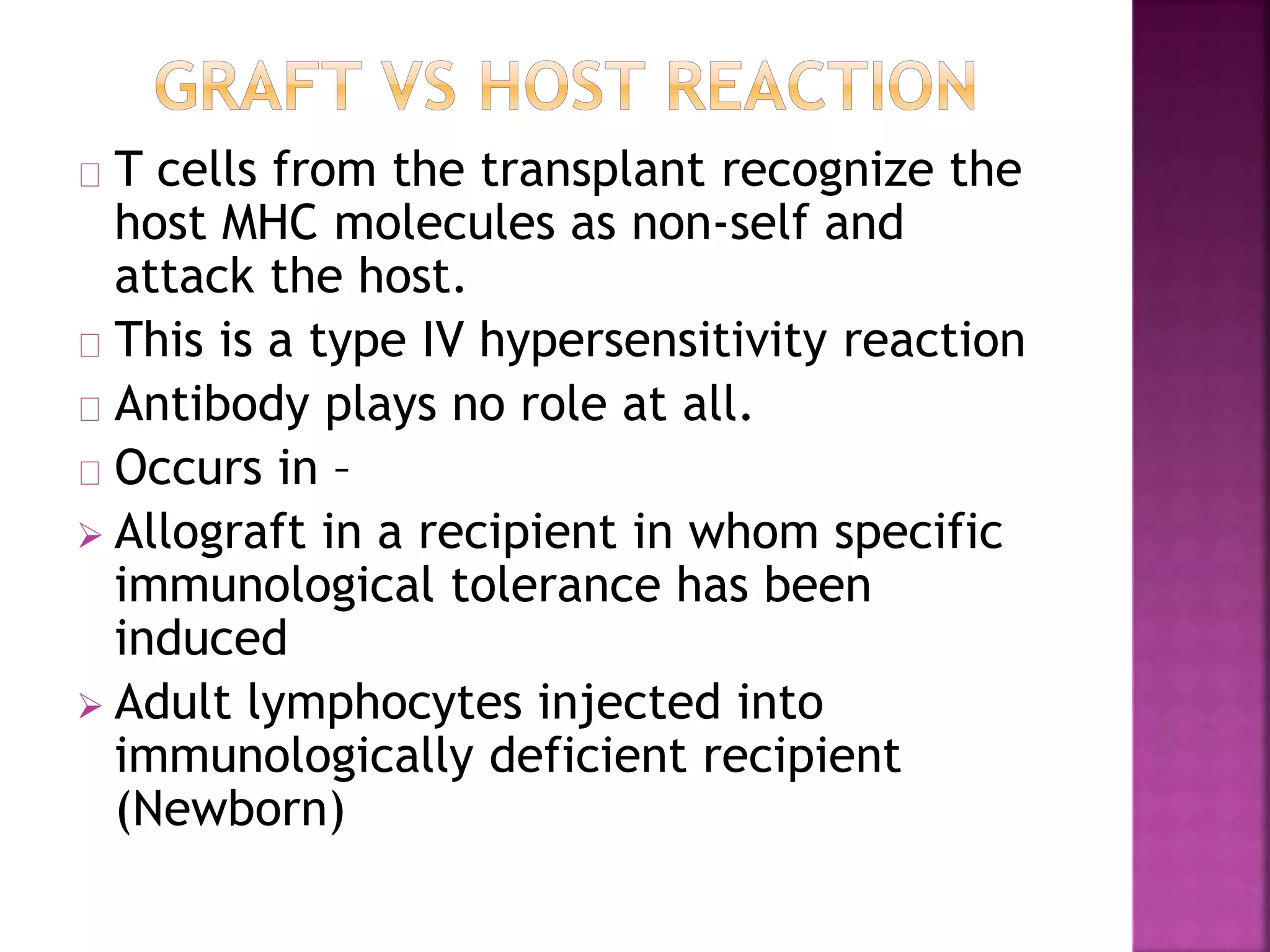
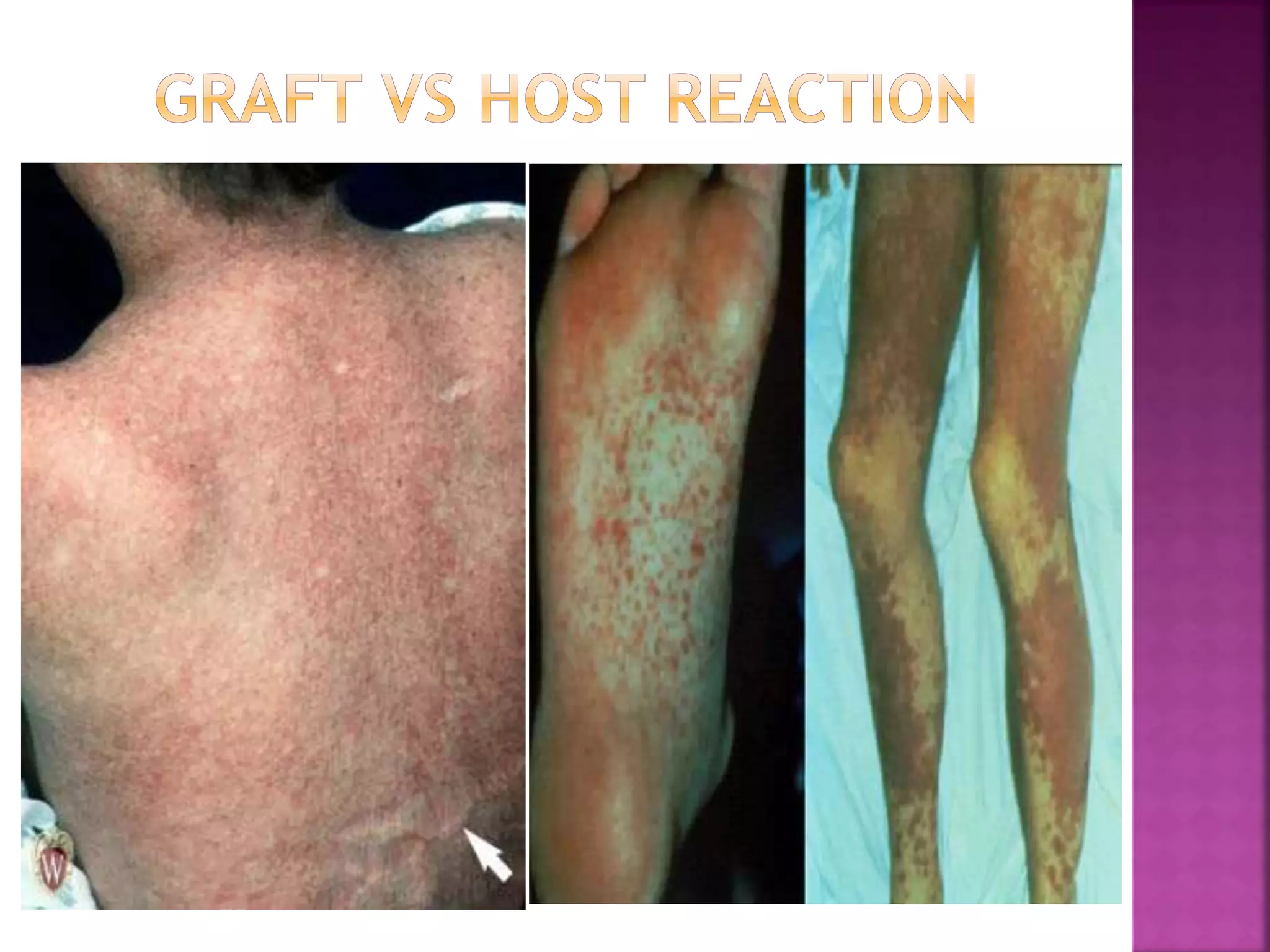
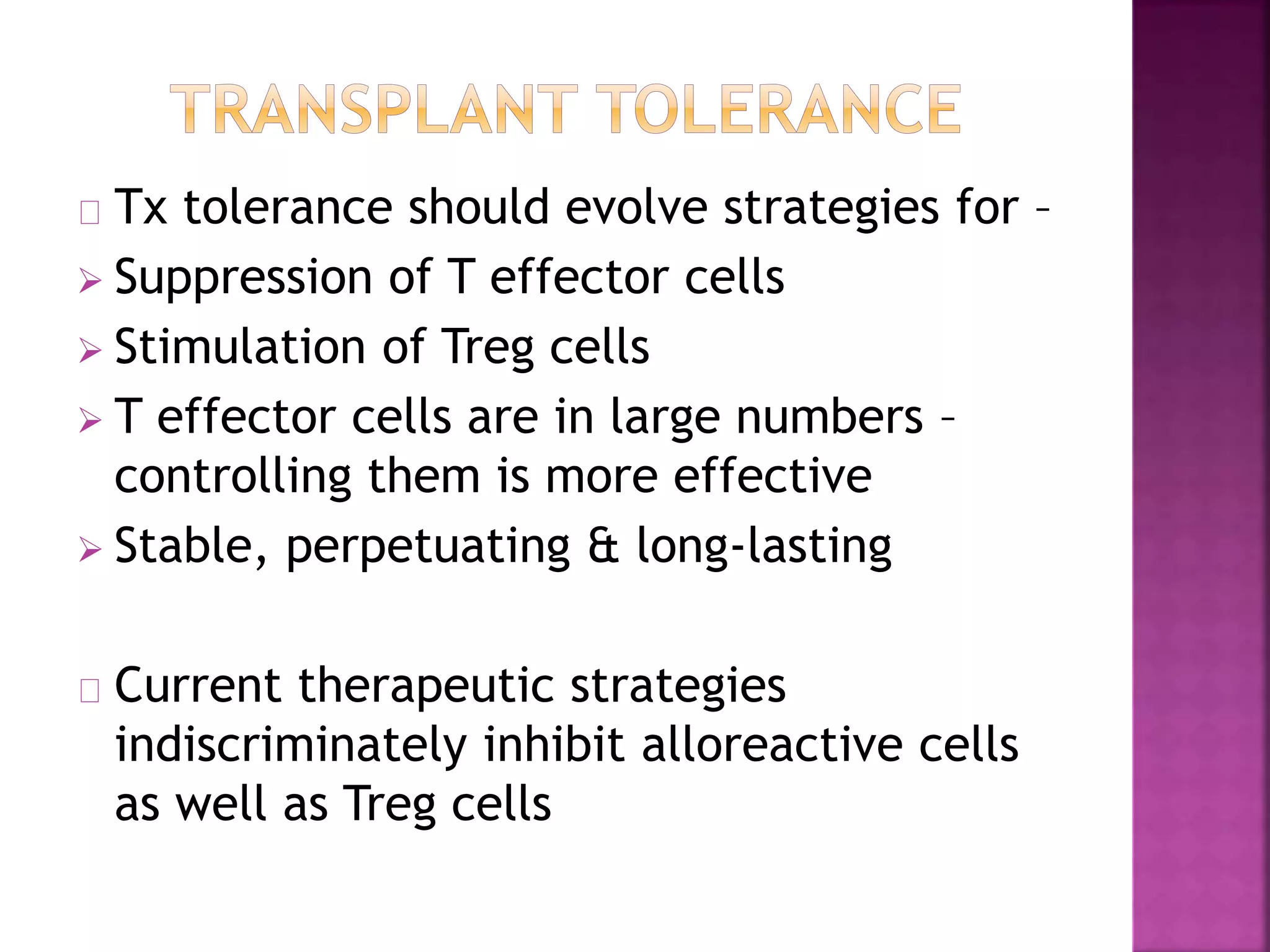
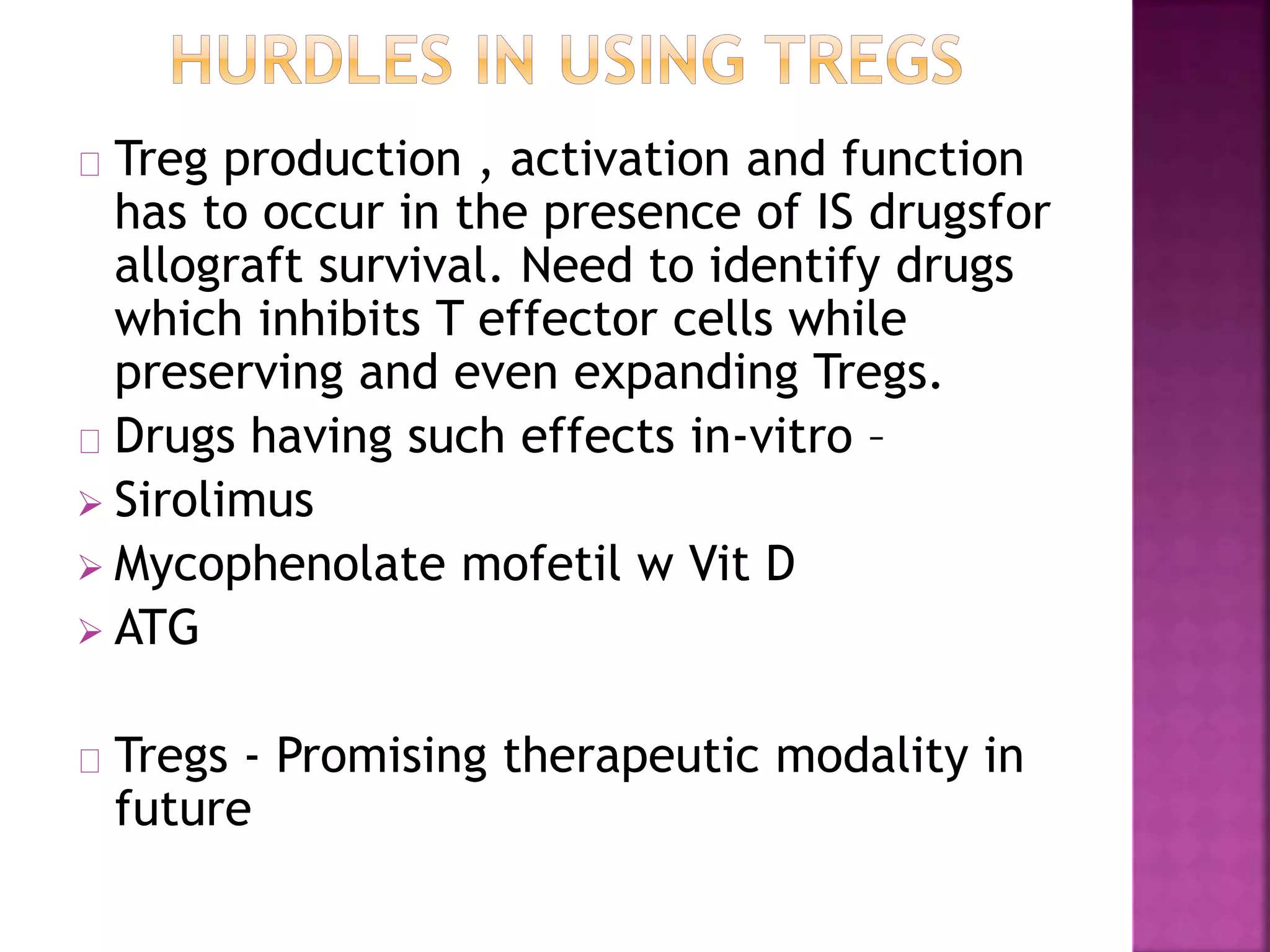
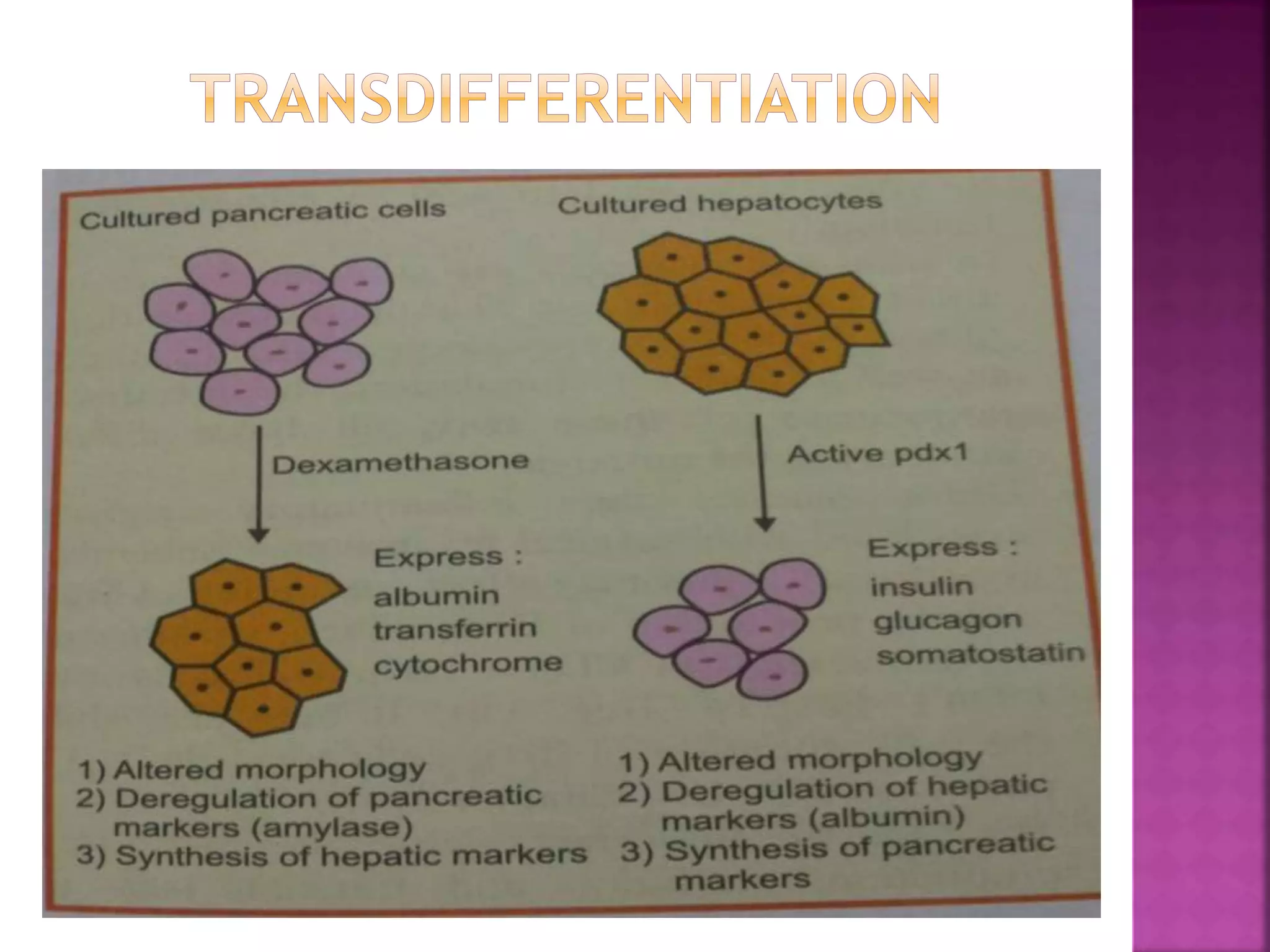
Recognition of transplanted cells is determined by polymorphic MHC genes inherited from both parents. Alloantigen elicit cell-mediated and humoral immune responses from components like antigen presenting cells, B cells, antibodies, and T cells. Cytokines also mediate graft rejection. HLA matching and immunosuppressive drugs are used to minimize rejection, but chronic rejection remains a problem. New methods using genomic analysis and RNA sequencing are being developed to better determine HLA type for transplantation matching.