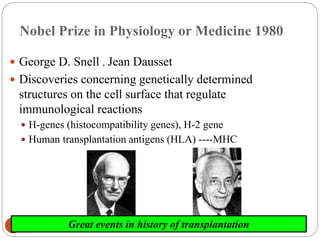
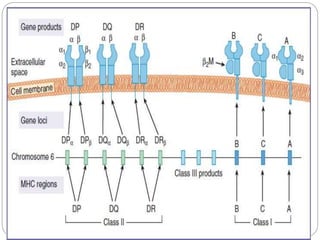
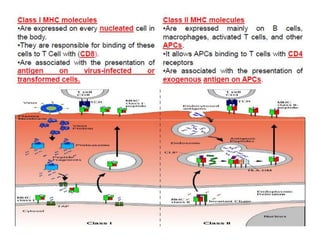
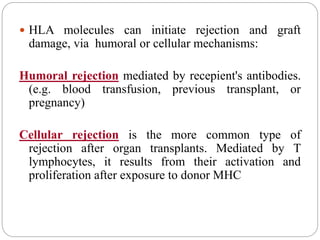
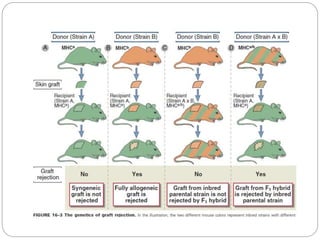
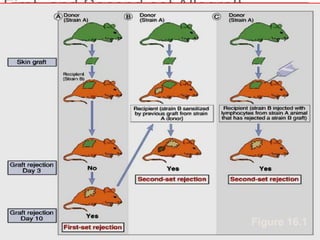
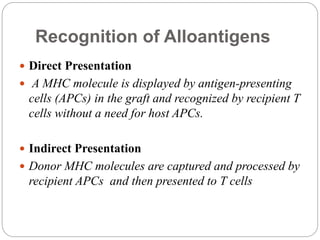
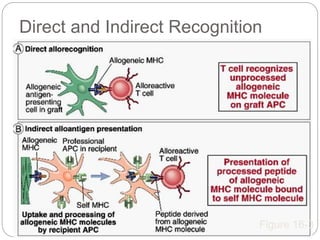
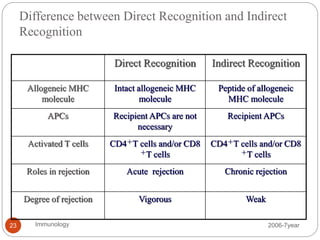
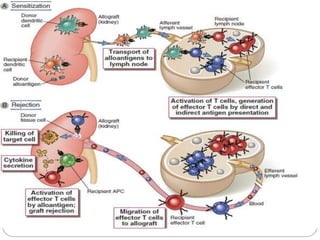
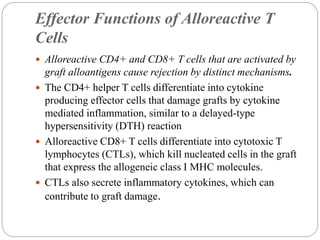
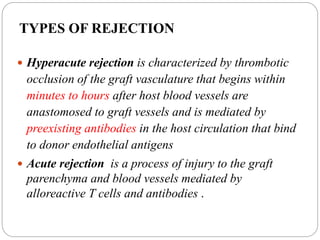
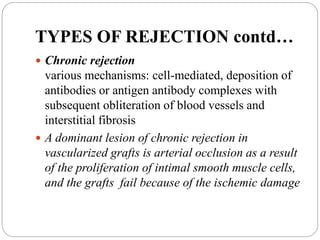
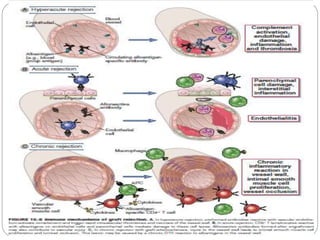
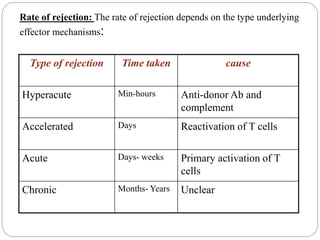
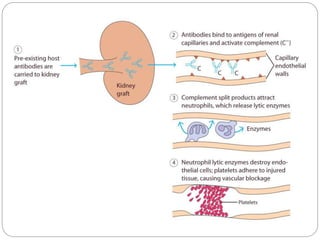
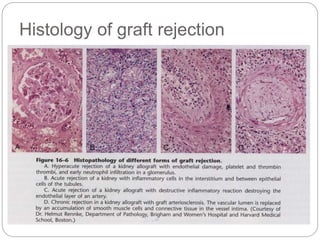
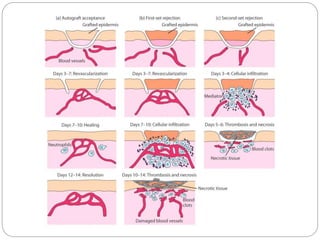
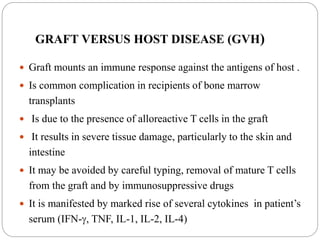
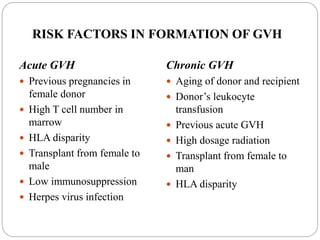
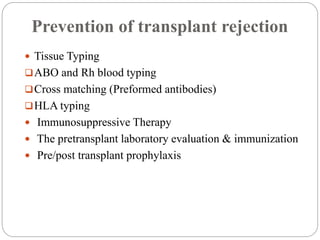
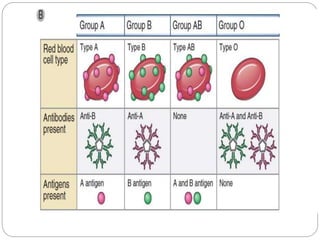
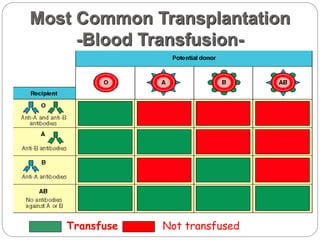
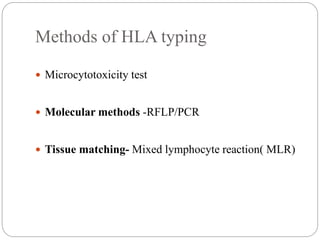
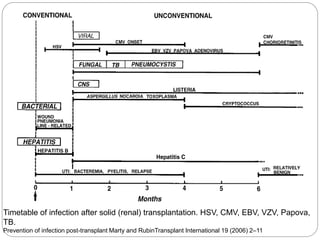
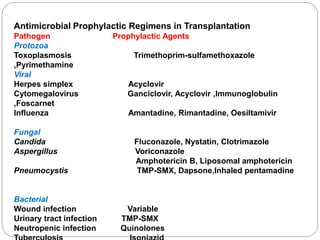
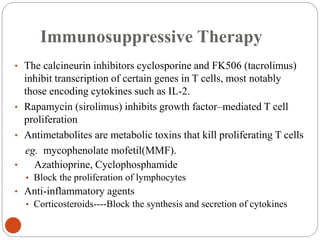
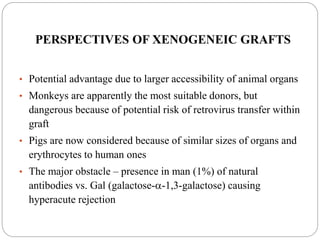
This document discusses the history and science of organ transplantation. It begins with a brief history, highlighting Nobel Prize-winning discoveries such as the first successful organ transplant between twins in 1954. It then covers key topics like the major histocompatibility complex and mechanisms of graft rejection, such as acute cellular rejection mediated by T cells. The types of transplantation are defined, from autologous to xenogeneic grafts. Prevention of rejection involves tissue typing, immunosuppression, and vaccination. Complications like graft-versus-host disease are also summarized.