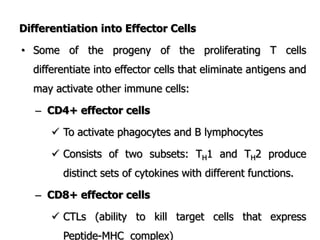
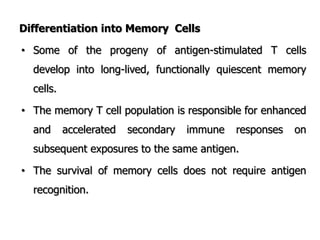
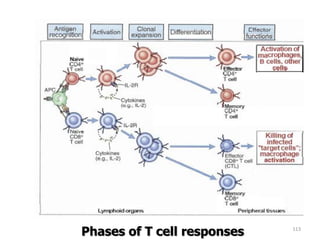
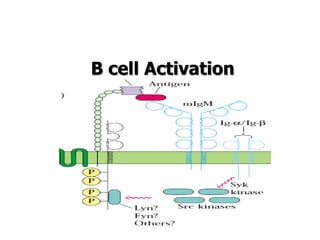
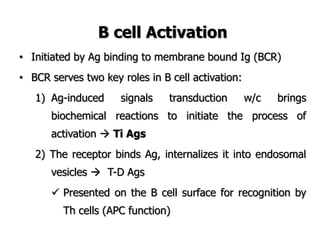
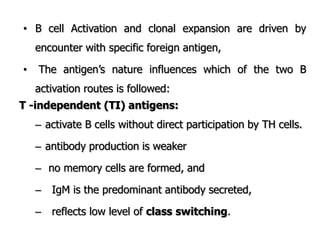
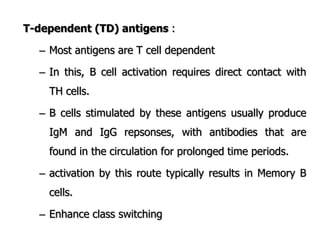
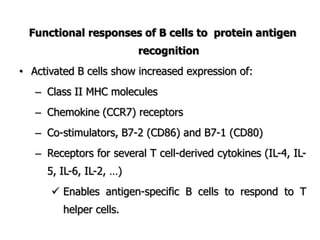
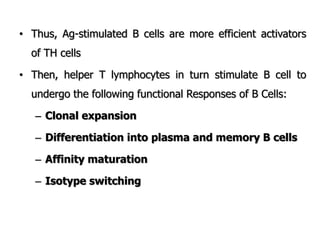
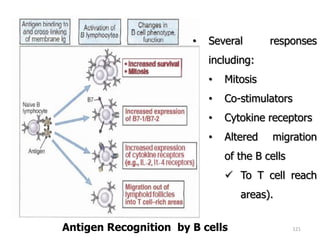
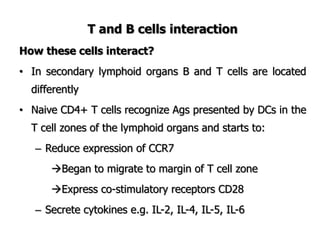
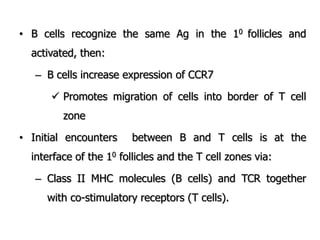
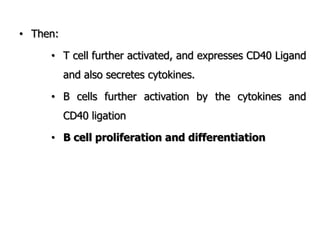
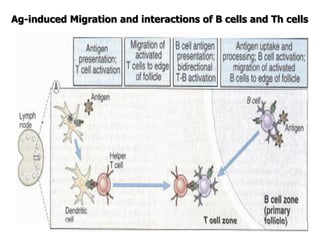
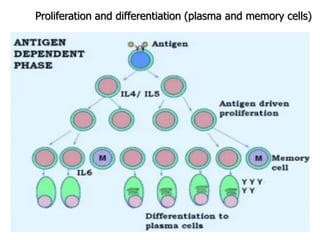
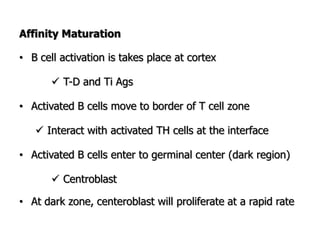
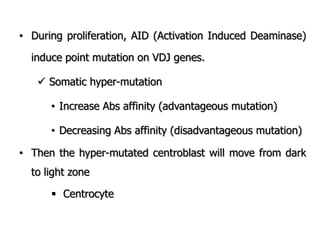
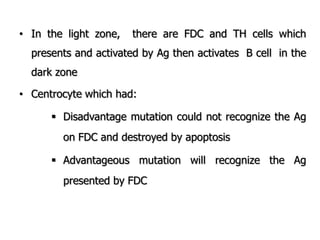
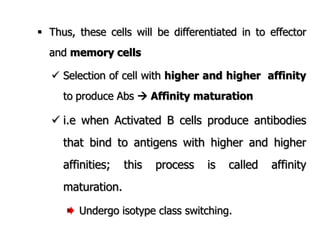
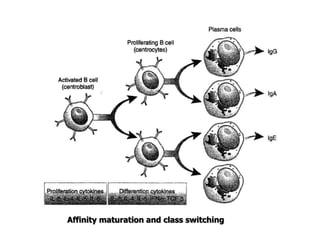
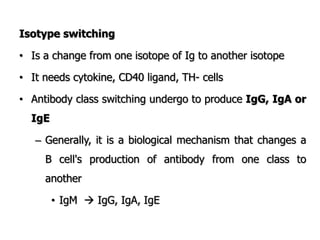
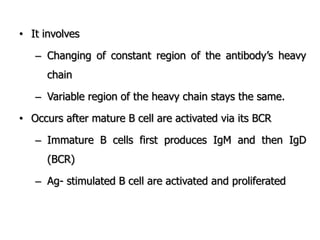
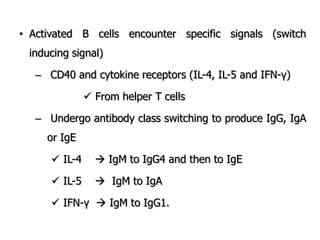
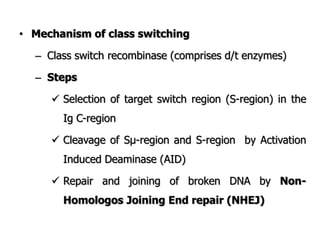
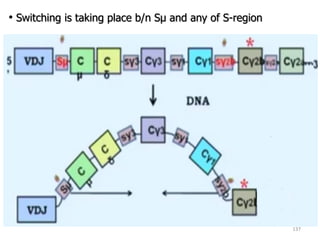
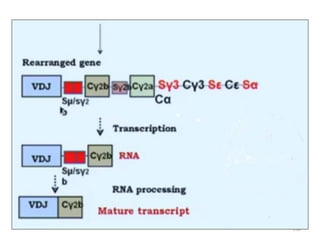
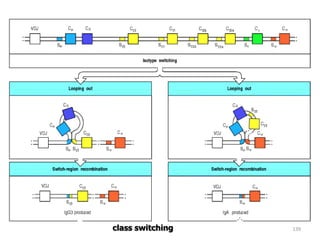
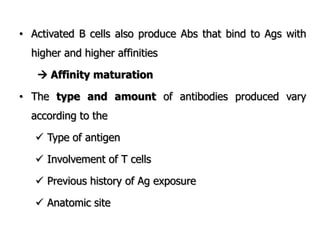
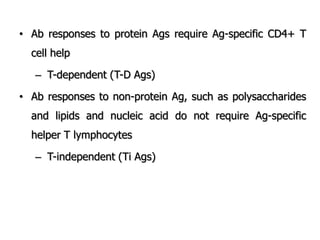
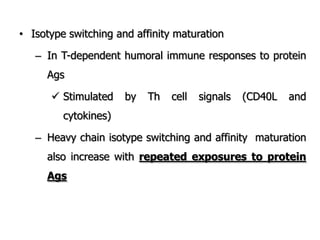
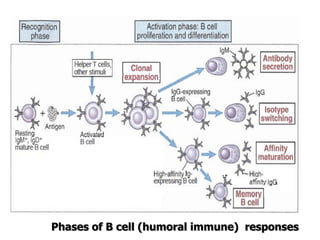
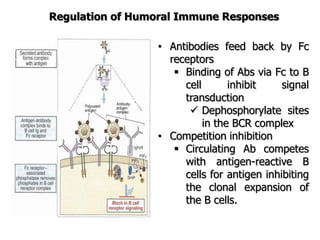
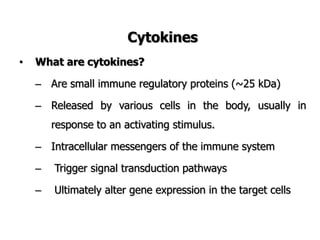
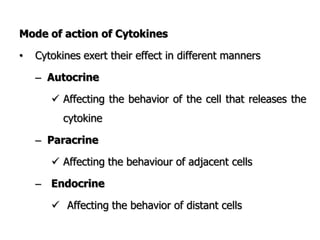
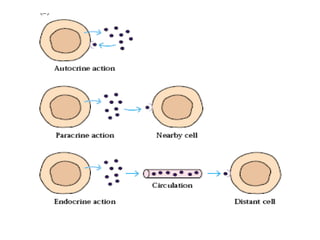
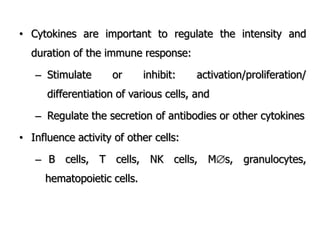
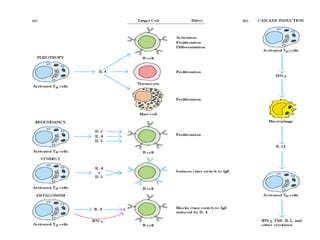
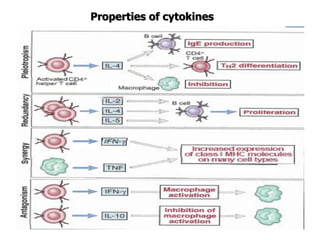
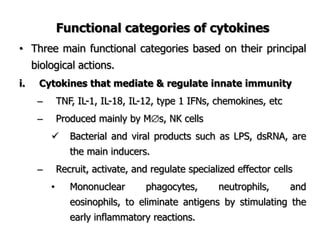
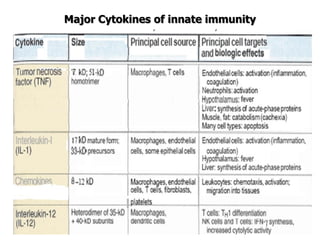
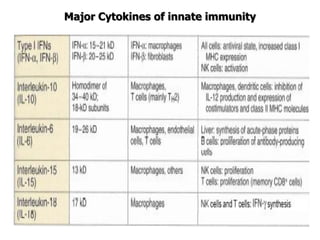
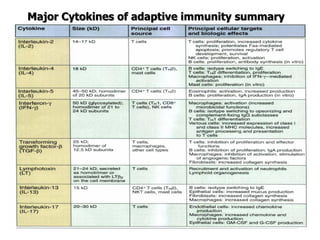
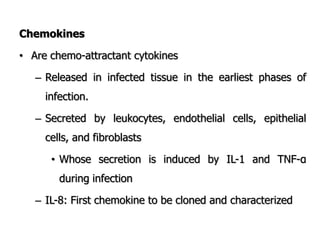
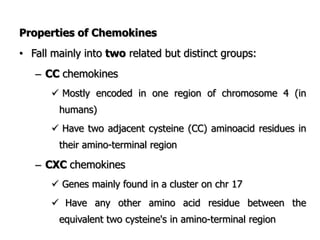
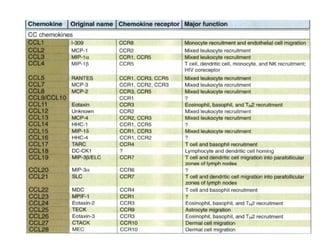
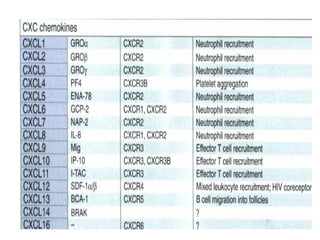
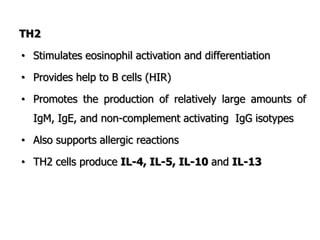
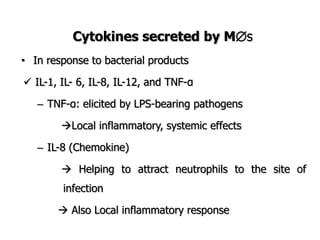
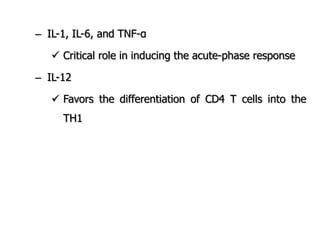
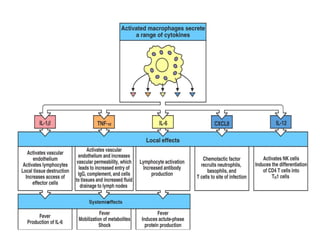
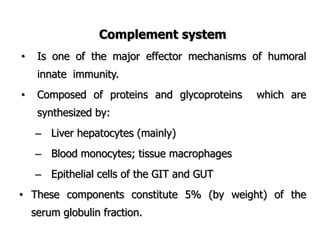
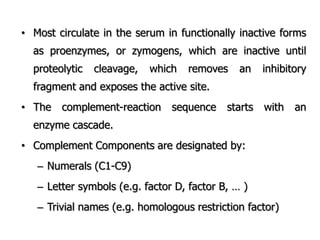
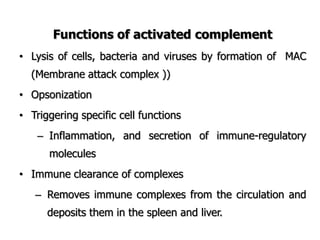
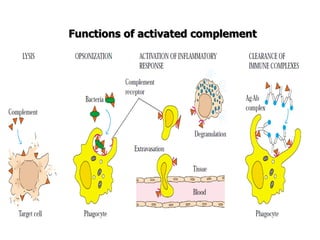
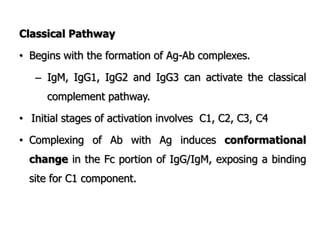
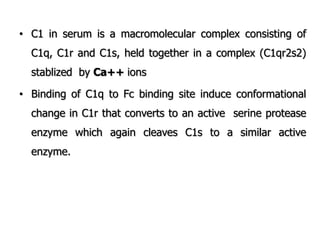
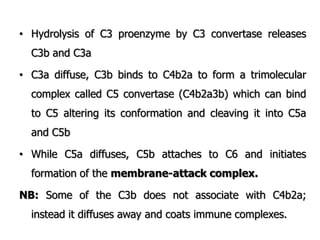
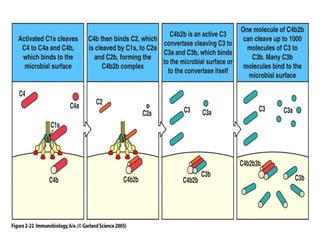
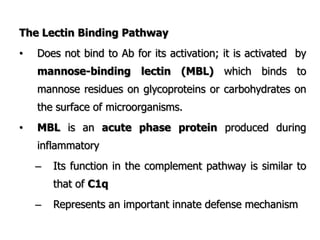
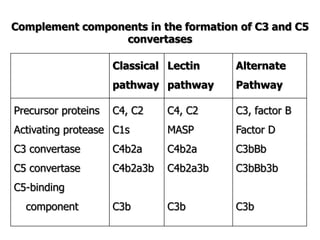
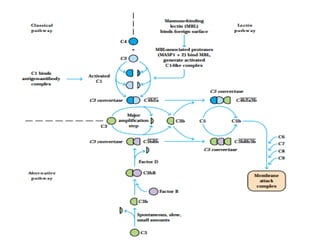
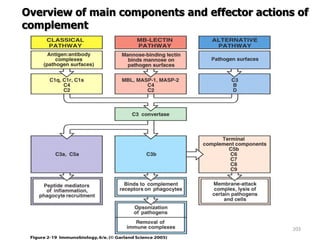
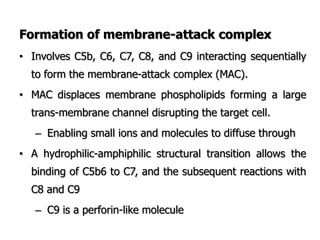
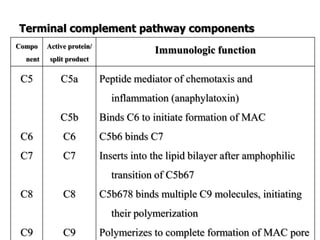
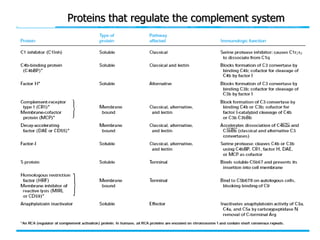
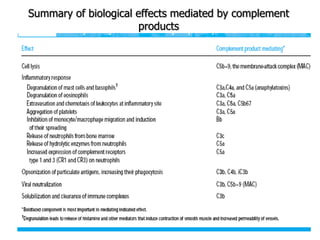
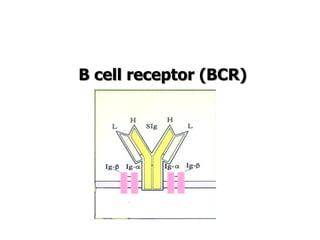
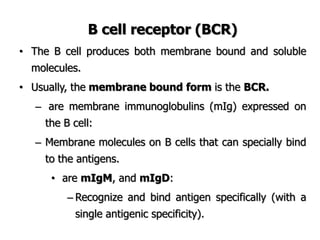
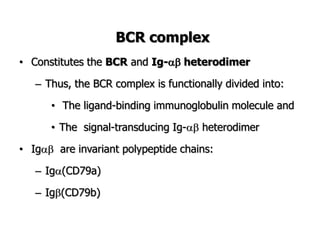
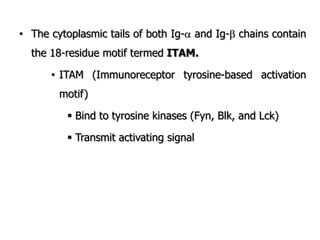
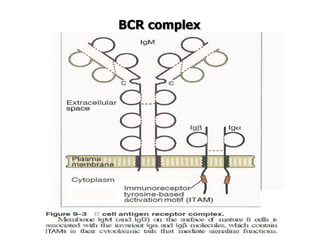
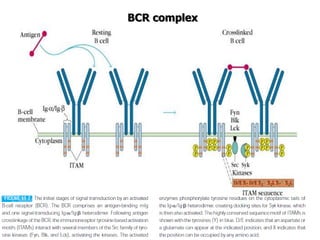
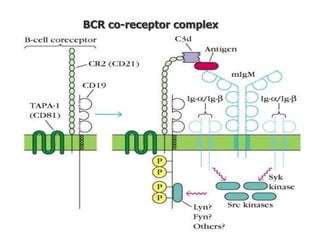
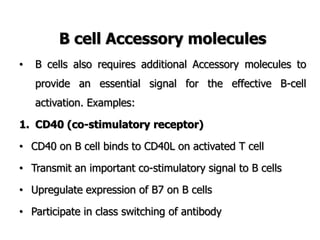
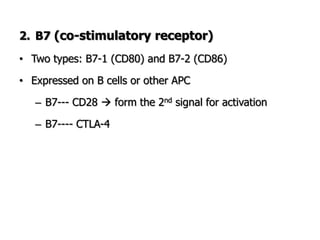
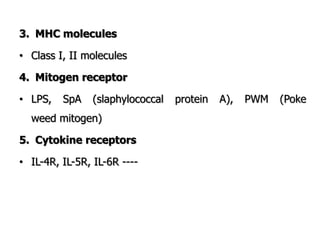
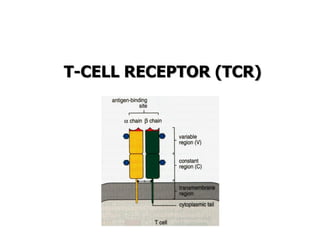
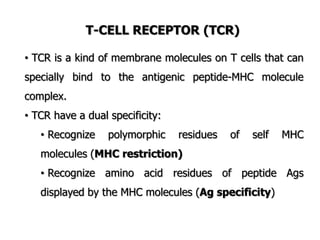
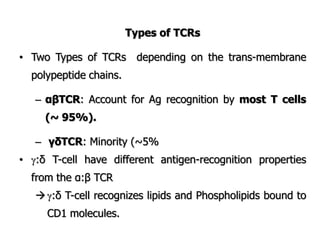
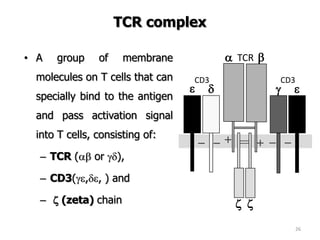
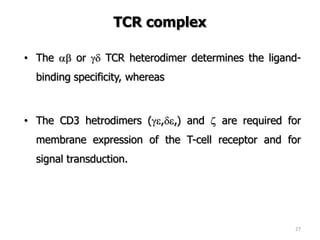
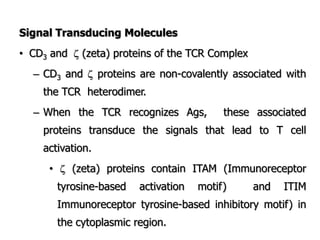
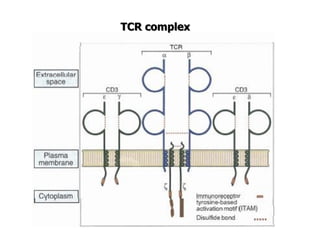
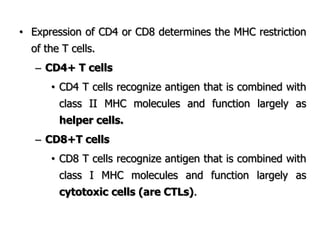
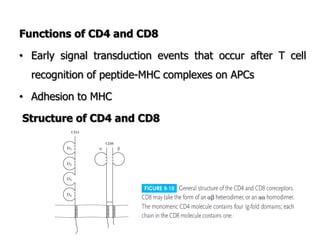
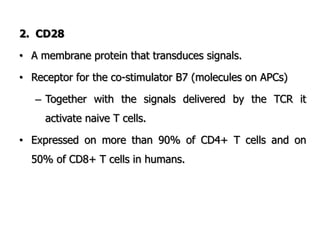
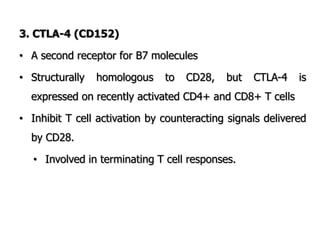
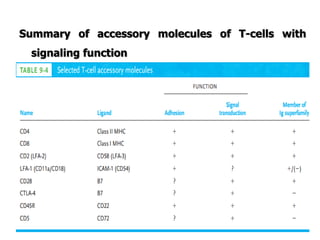
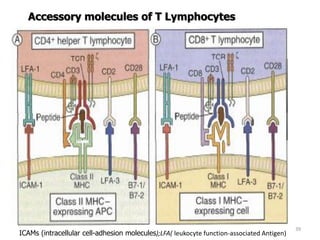
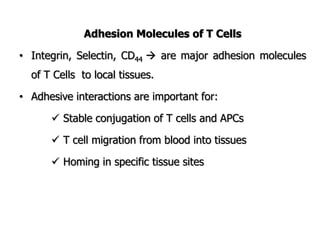
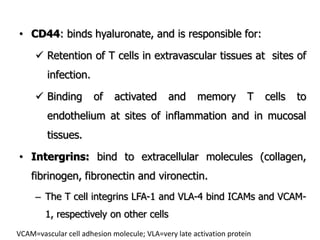
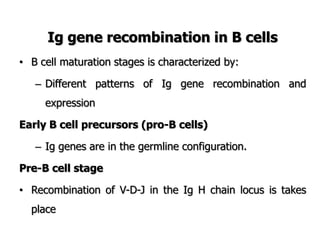
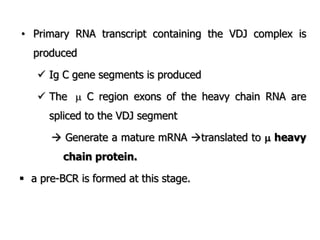
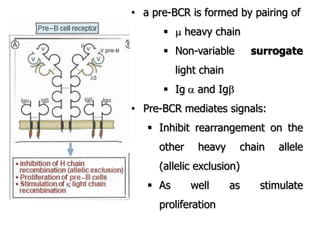
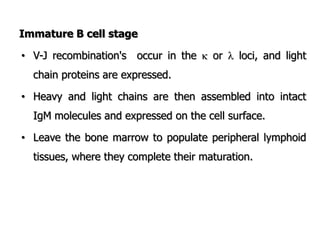
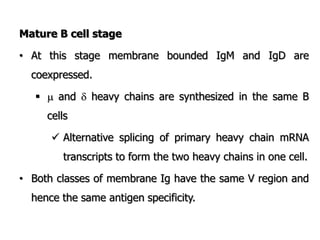
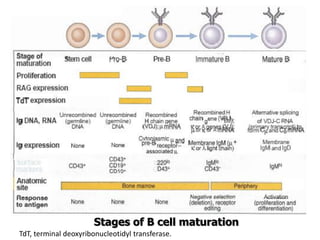
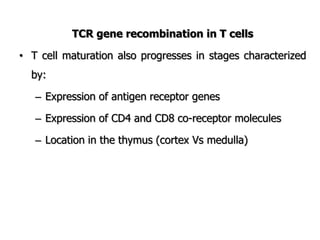
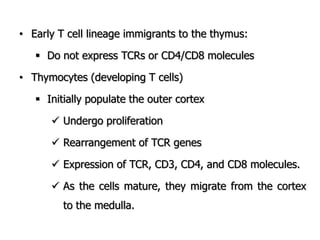
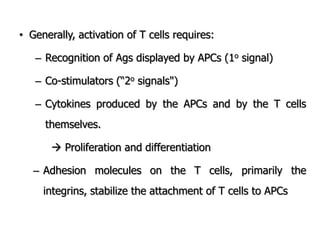
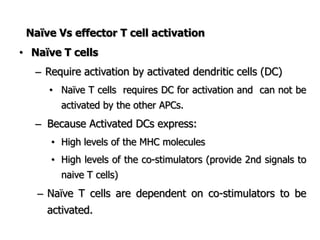
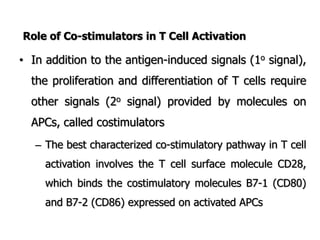
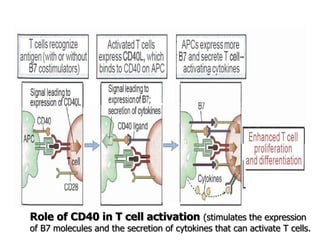
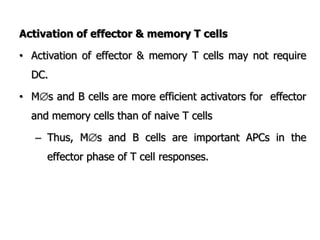
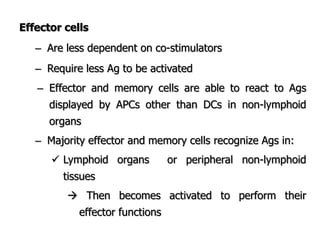
The document discusses T and B cell receptors. B cell receptors (BCRs) are membrane immunoglobulins expressed on B cells that recognize and bind antigen specifically. The BCR complex includes the immunoglobulin molecule and Ig-αβ heterodimer, which contains immunoreceptor tyrosine-based activation motifs that transmit activating signals. B cells also require coreceptor and accessory molecules like CD19, CD21, and CD40 to provide additional activation signals. T cell receptors (TCRs) recognize antigenic peptides bound to MHC molecules. The TCR complex includes the TCR heterodimer and CD3 and ζ chains that transduce signals. Accessory molecules like CD4, CD8, CD28







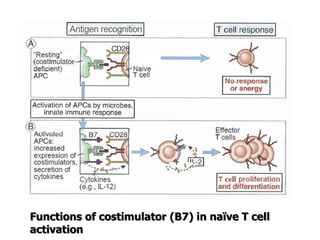
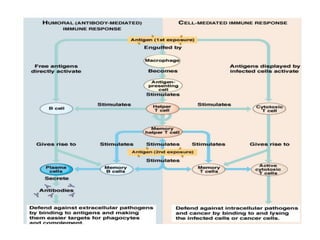
![– Effector T cells of the CD4+helper subset express
membrane molecules and secrete cytokines that
activate:
Ms to kill phagocytized microbes
B cells to differentiate into cells that secrete Abs
Humoral immunity
– Effector cells of the CD8+ subset [CD8+ cytolytic T
lymphocytes (CTLs)] kill infected cells and tumor cells.
Cell mediated immunity](https://image.slidesharecdn.com/immunology-180903154153/85/Immunology-1-106-320.jpg)