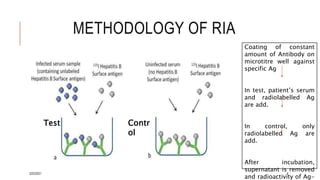
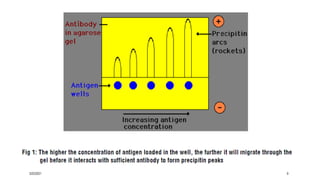
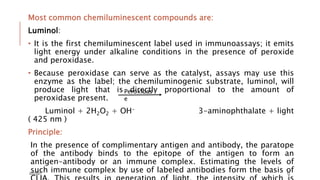
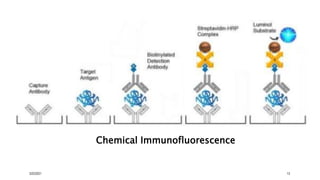
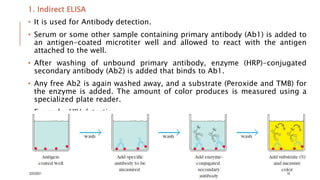
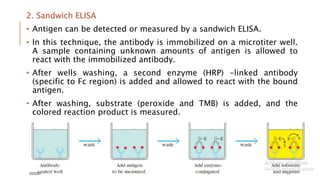
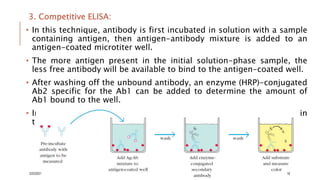
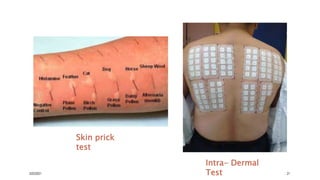
This document provides an overview of various immunological techniques including radioimmunoassay (RIA), rocket electroimmunodiffusion, chemical immunofluorescence, enzyme-linked immunosorbent assay (ELISA), and allergen testing. It describes the basic principles, methodologies, applications, advantages, and disadvantages of each technique. Radioisotopes commonly used in RIA and the steps of the RIA methodology are detailed. The document also discusses specific chemiluminescent compounds, types of ELISAs, and in vivo and in vitro allergen testing methods.