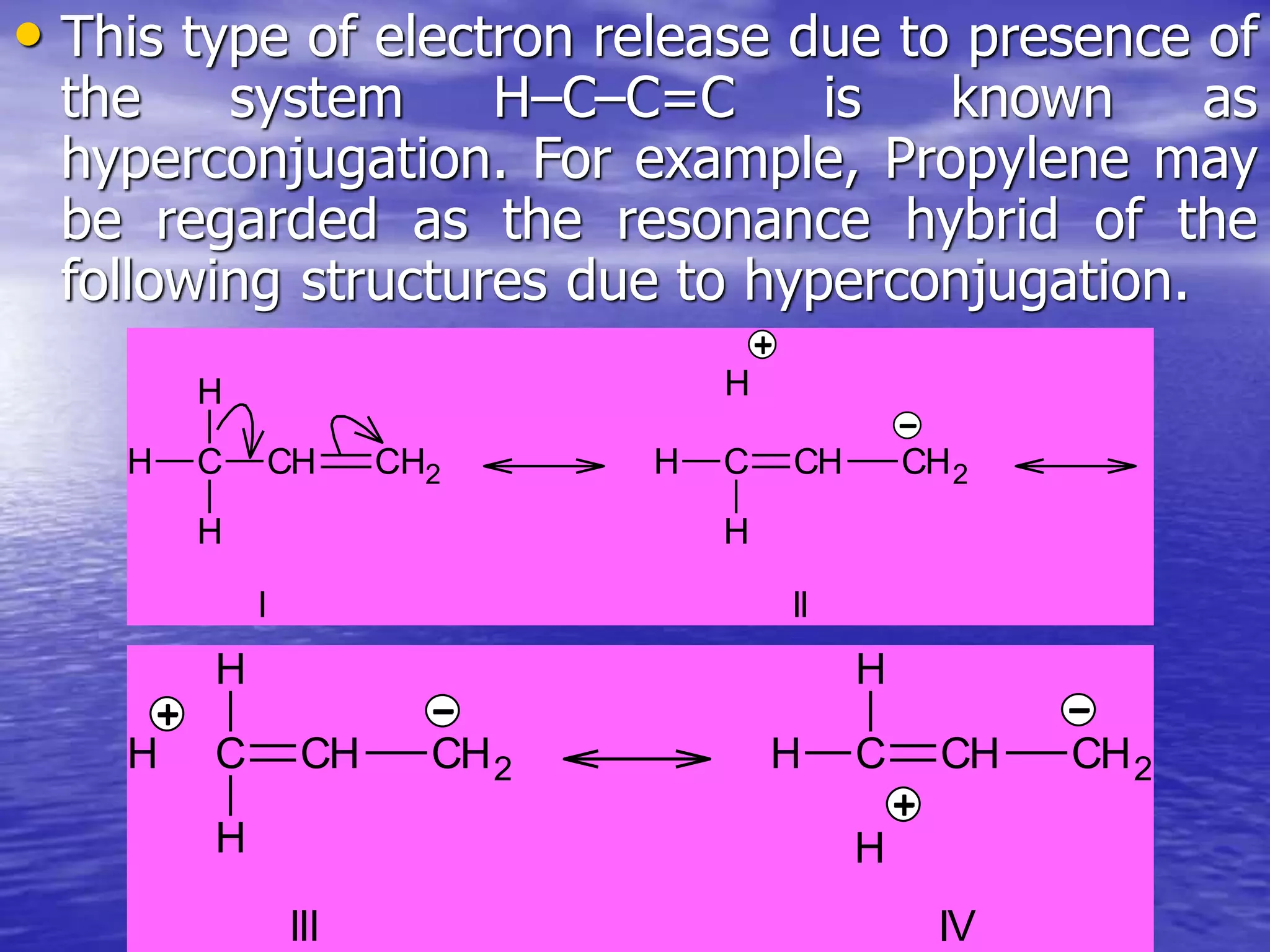
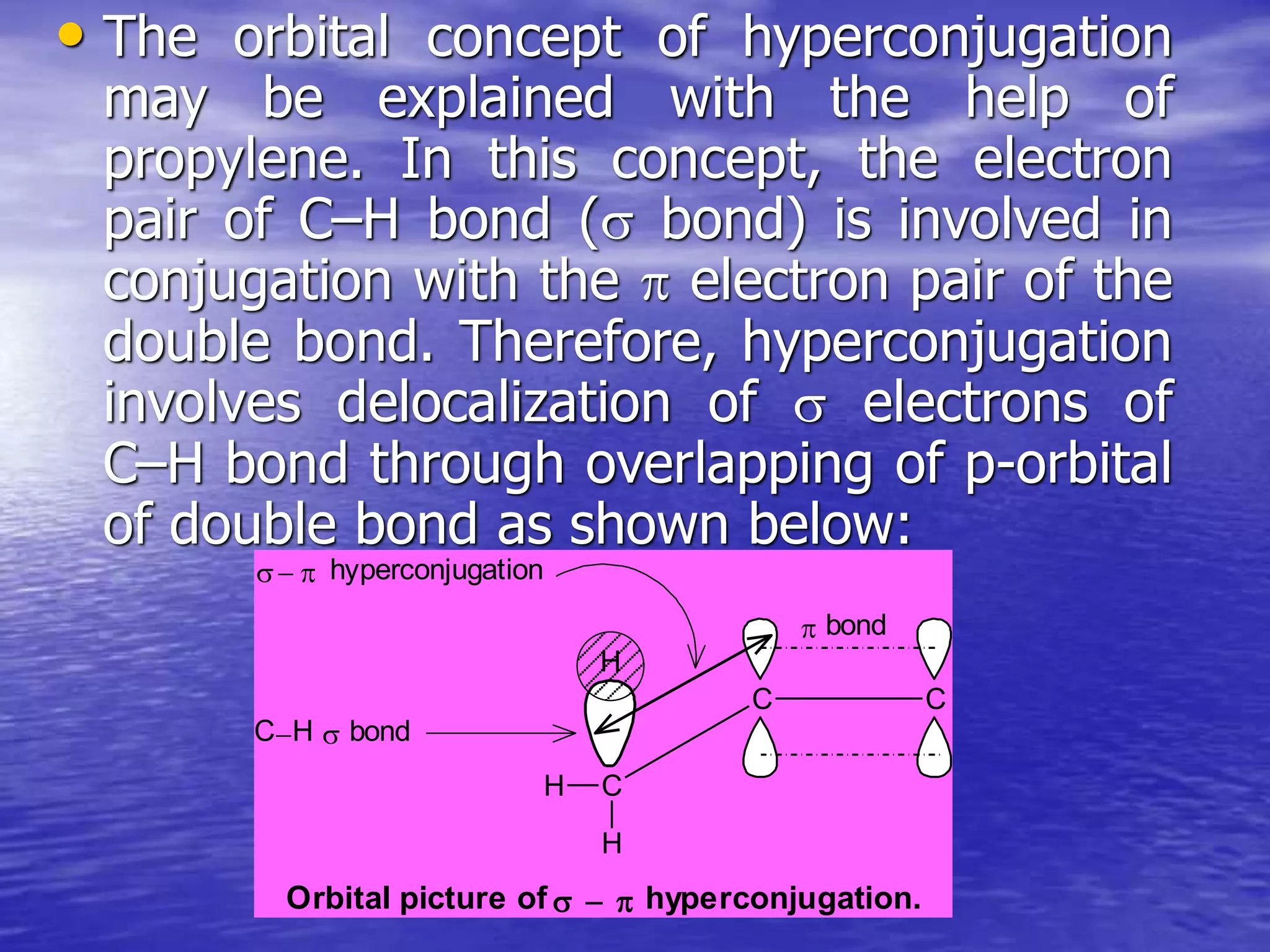
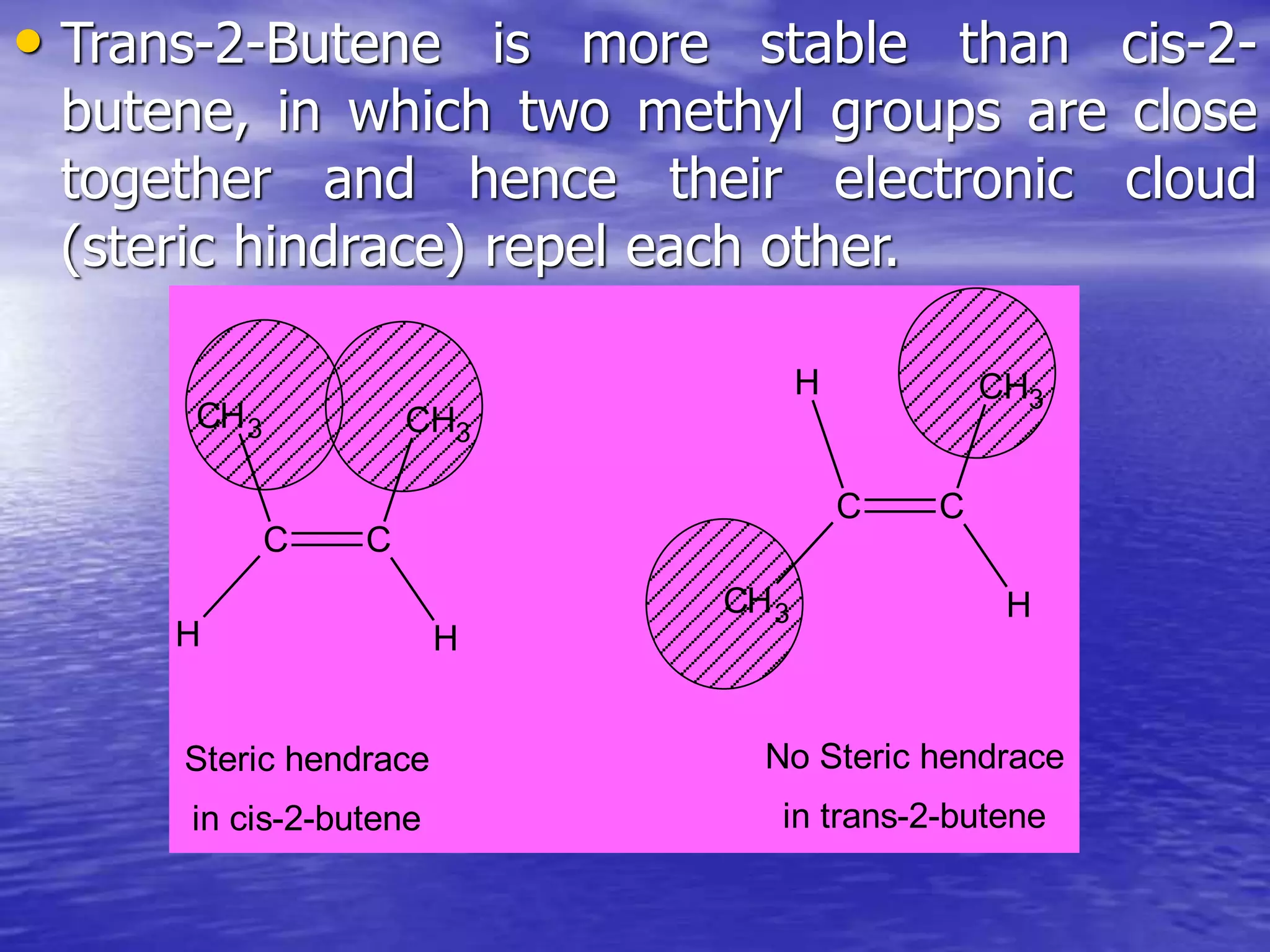
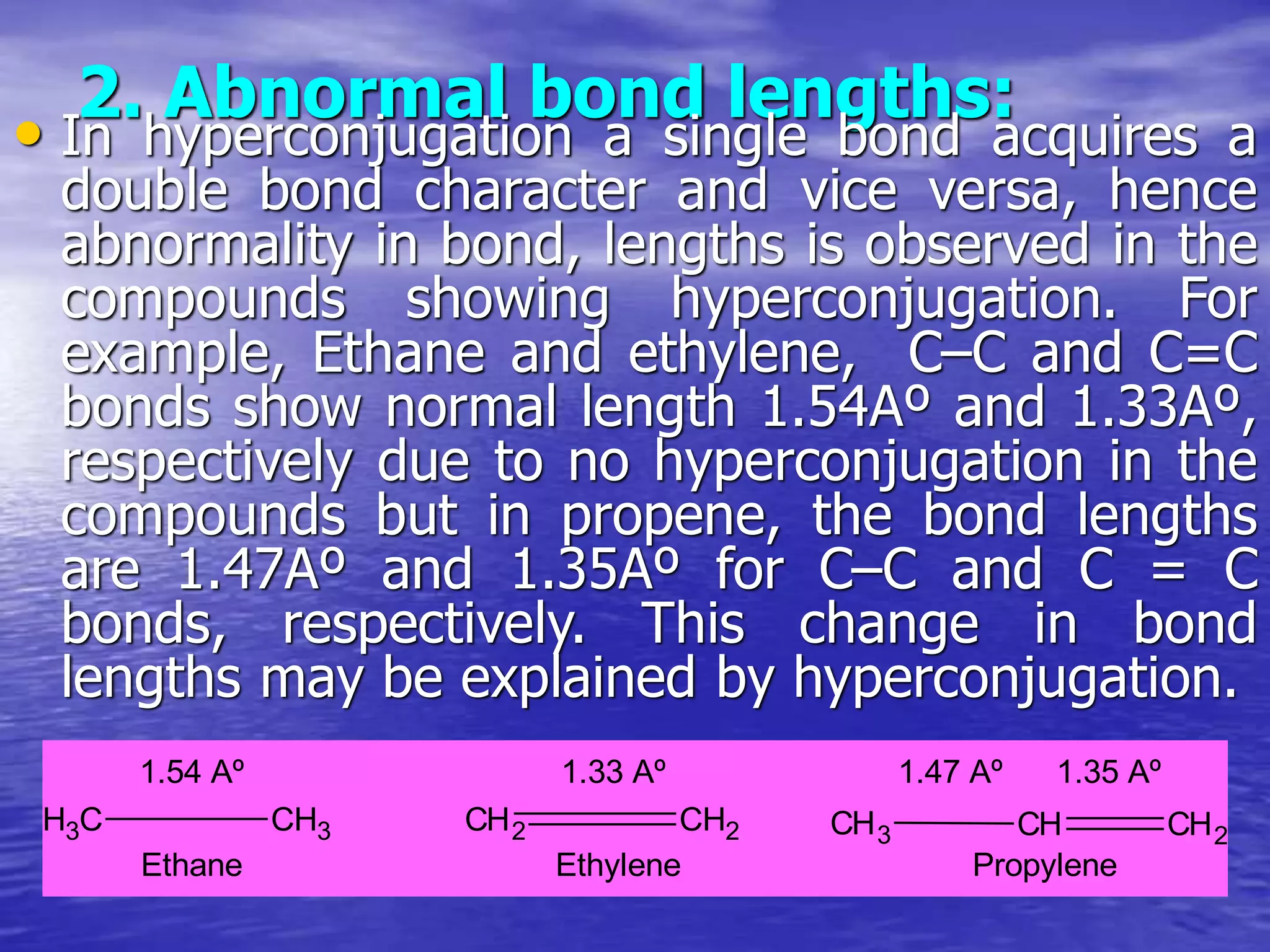
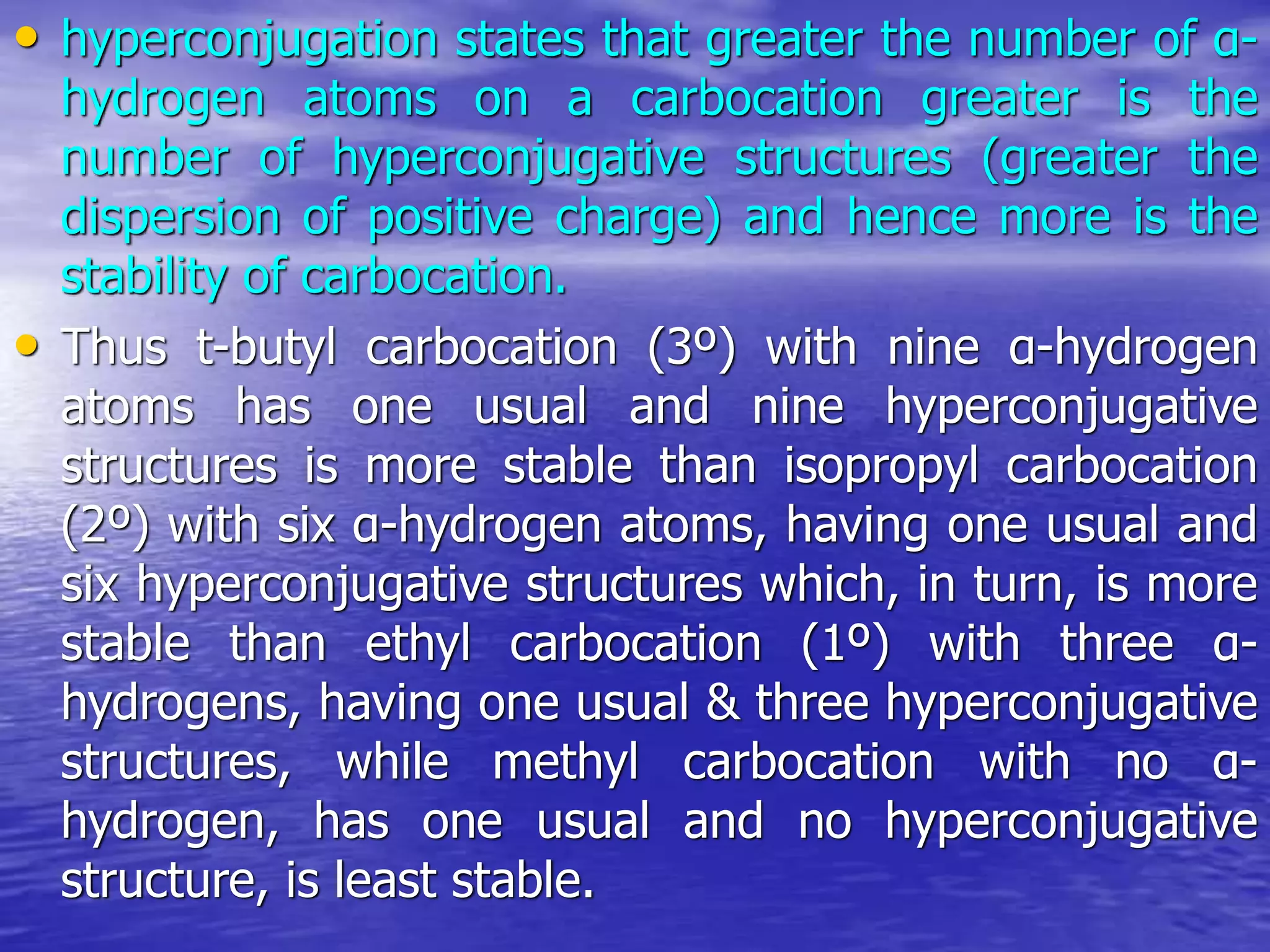
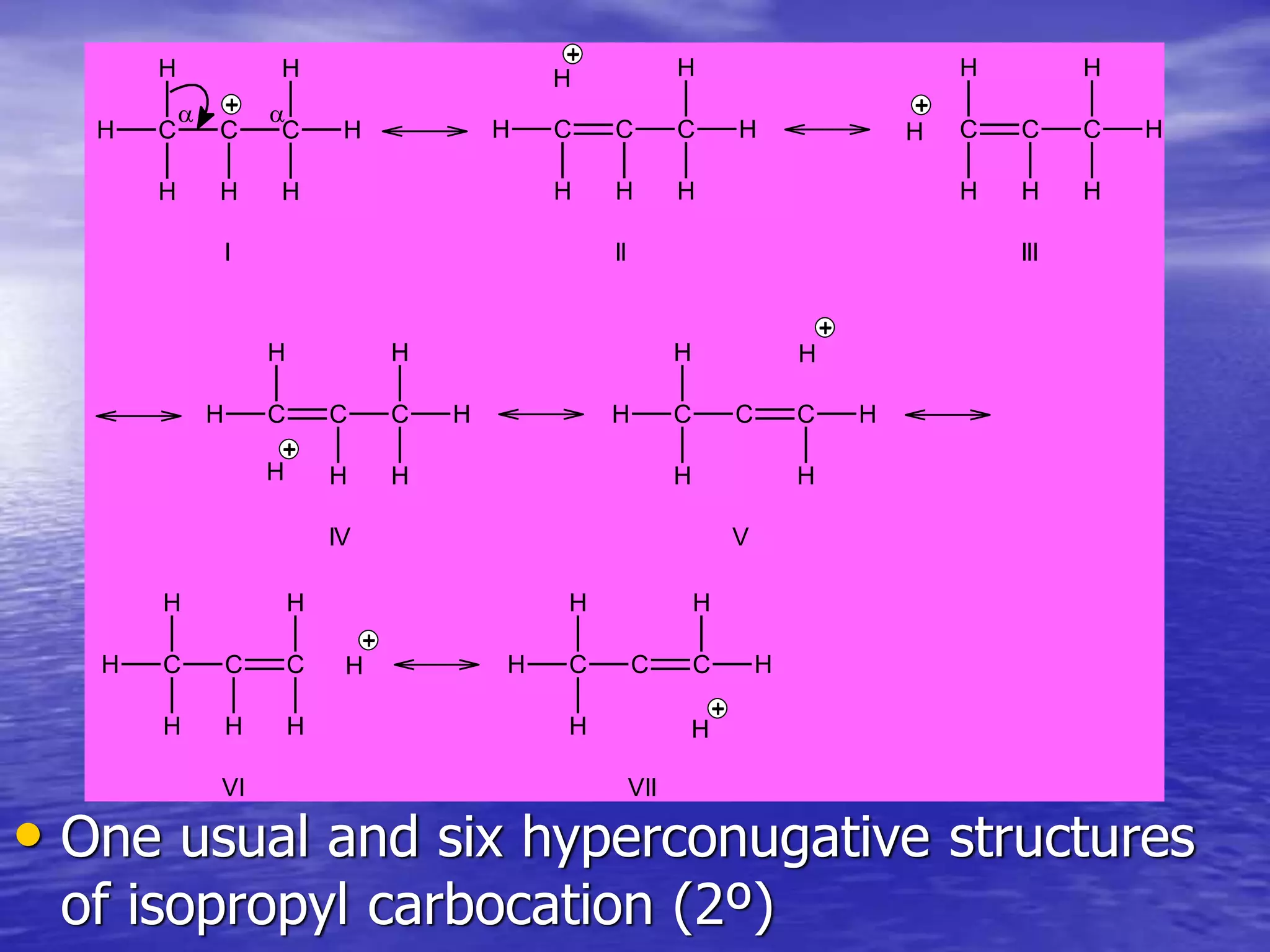
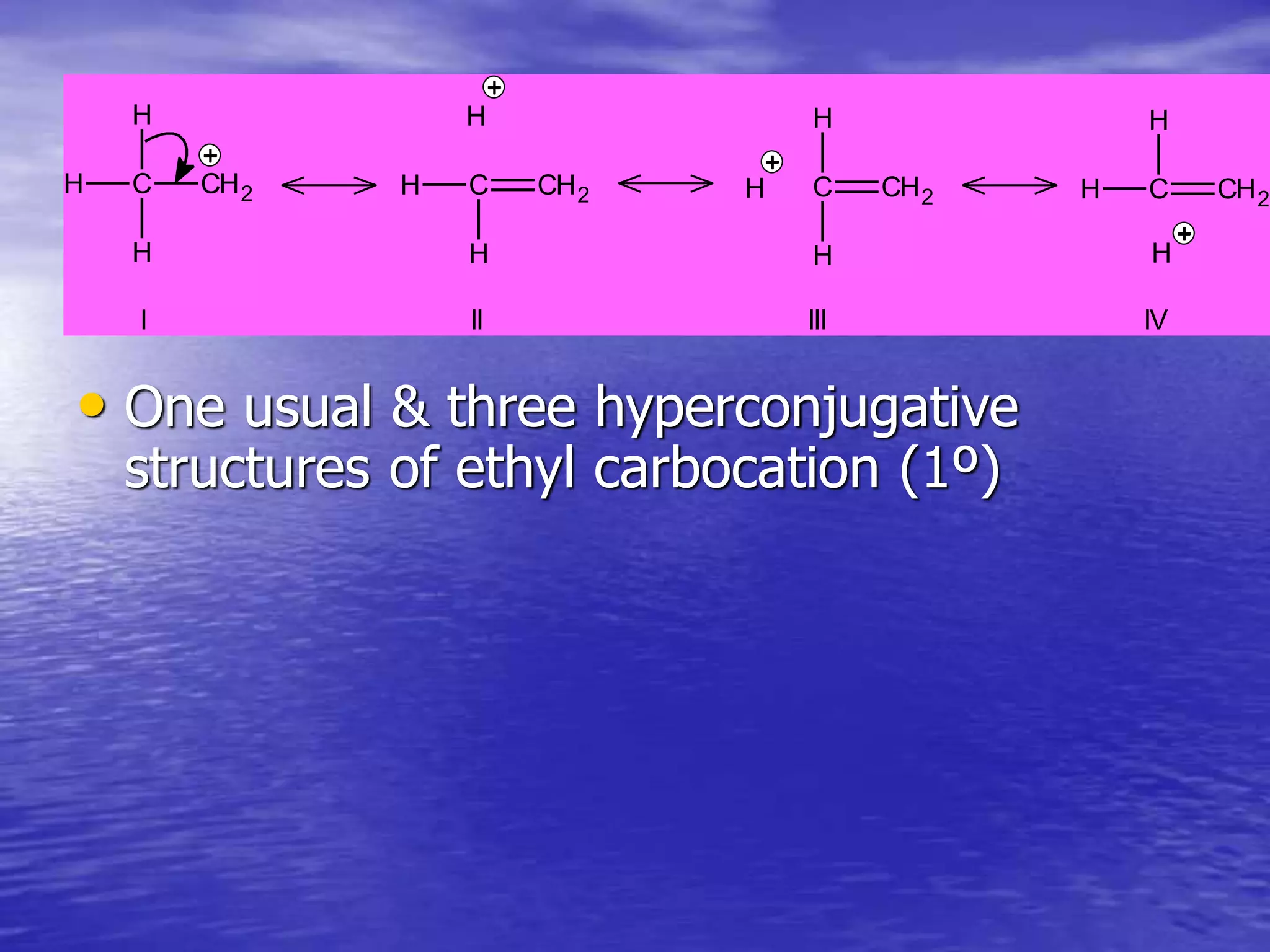
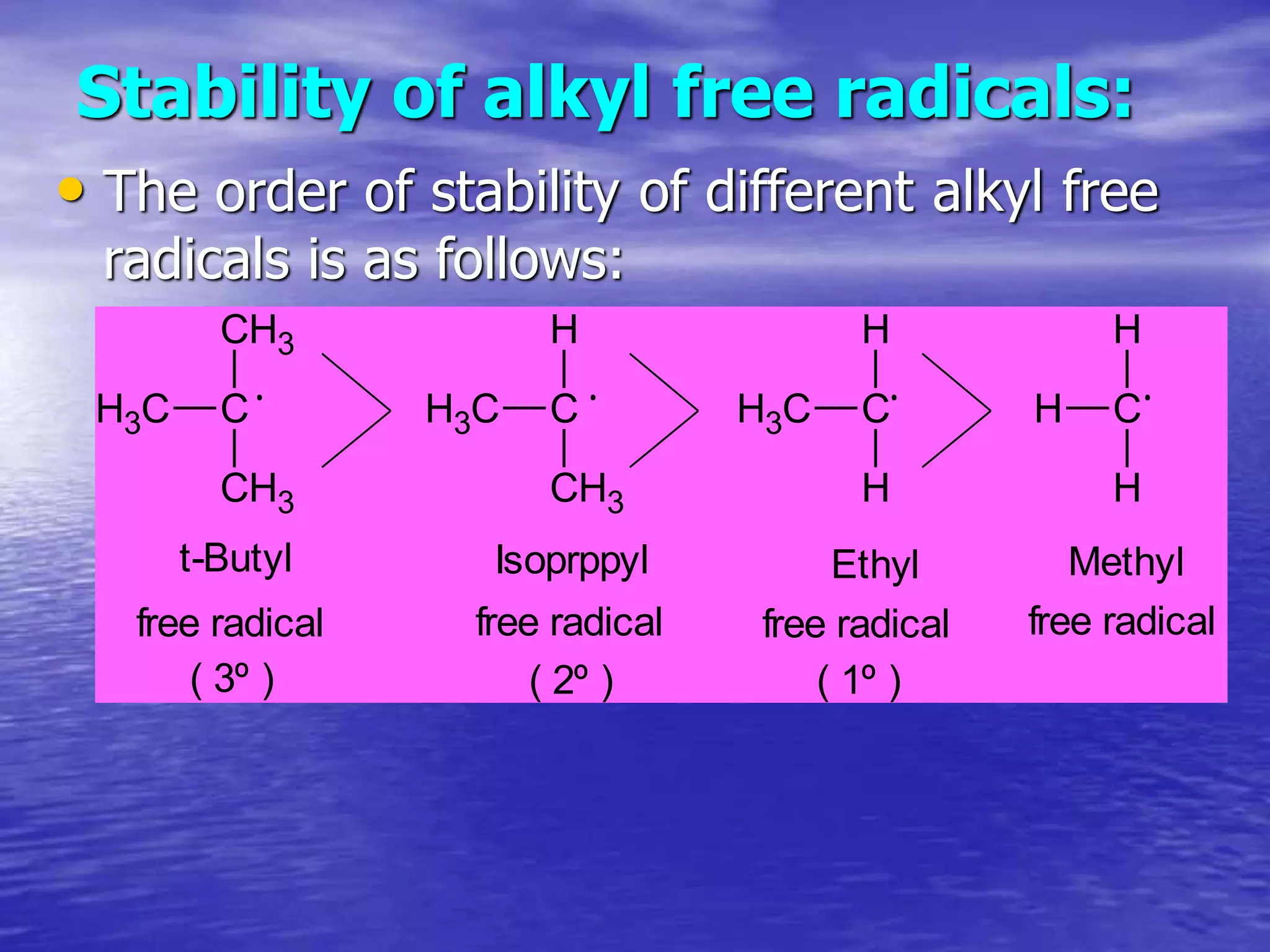
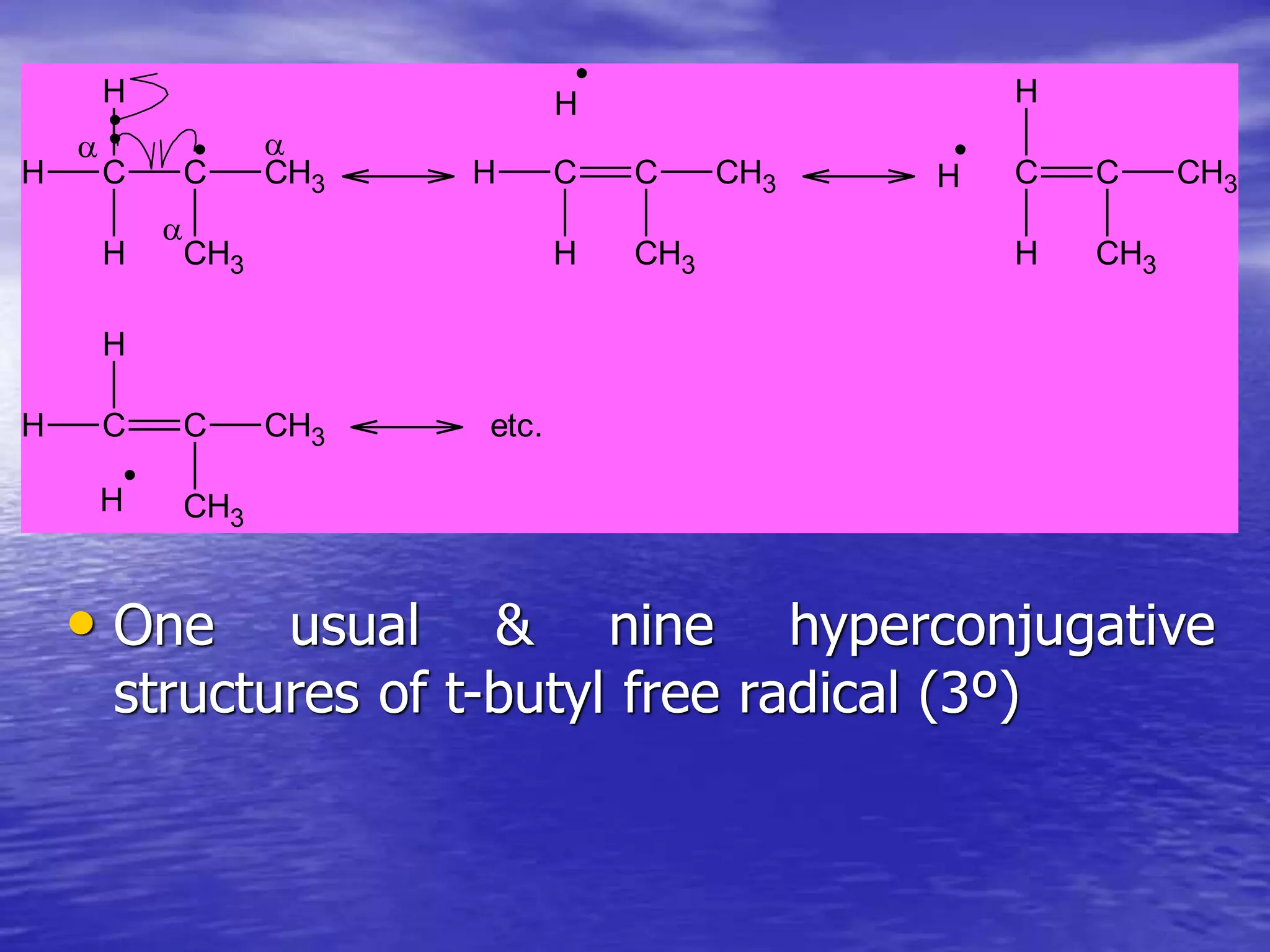
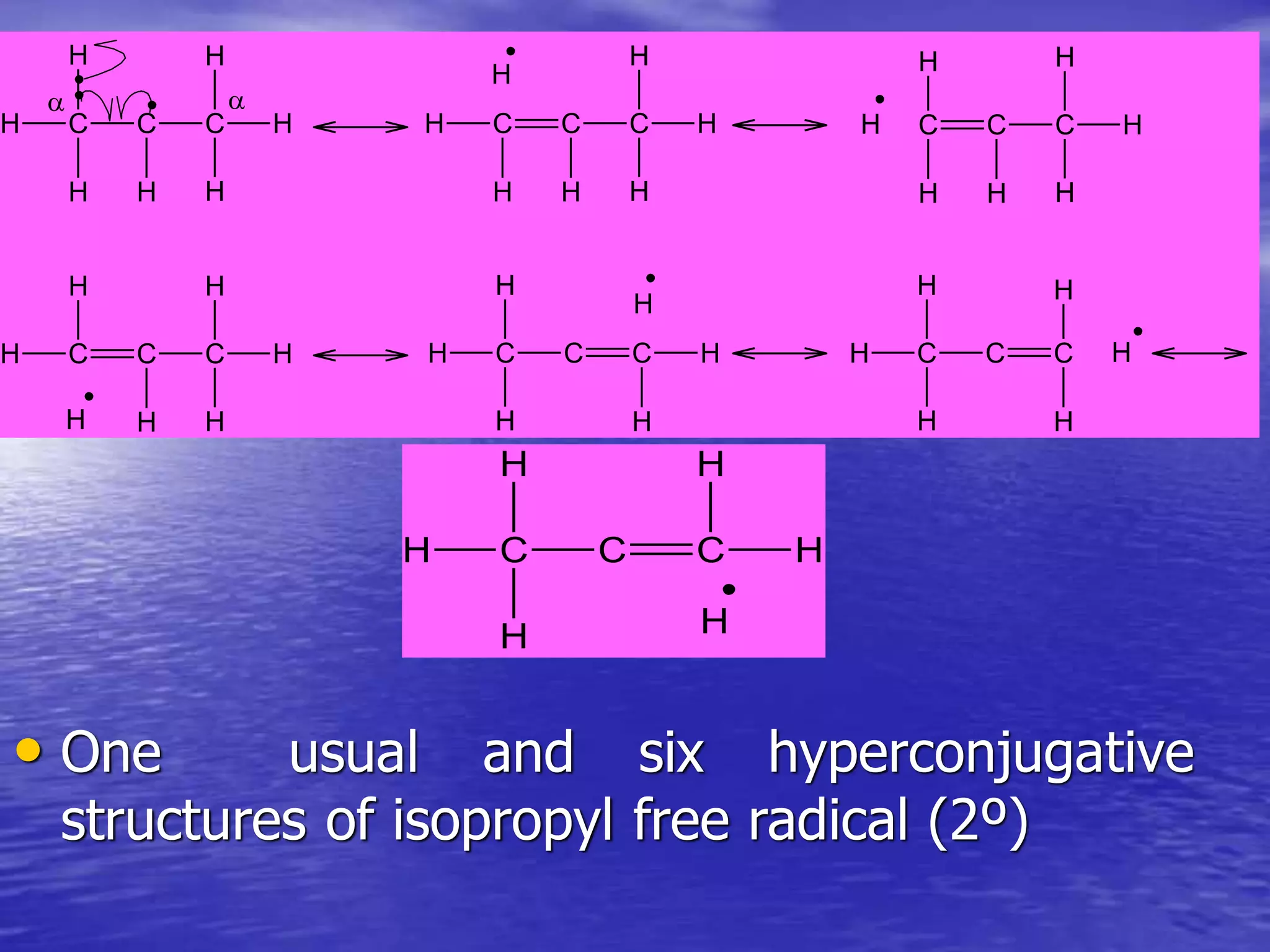
Hyperconjugation allows for the delocalization of electrons from C-H sigma bonds into adjacent pi systems. This increases the stability of compounds that can undergo hyperconjugation such as alkenes, carbocations, and alkyl radicals. The number of contributing hyperconjugative structures correlates with increased stability, explaining trends like the greater stability of tertiary carbocations and radicals compared to secondary or primary ones. Hyperconjugation also influences bond lengths and the orientation of substitution in aromatic compounds.