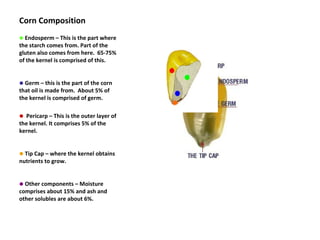
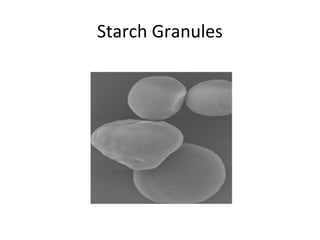
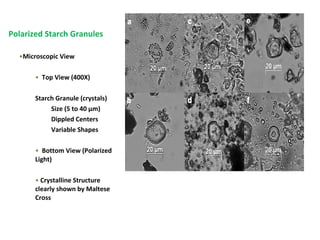
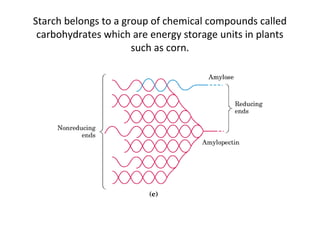
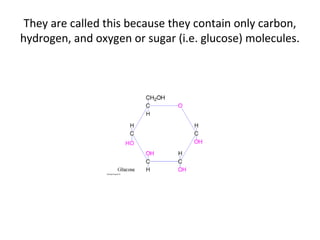
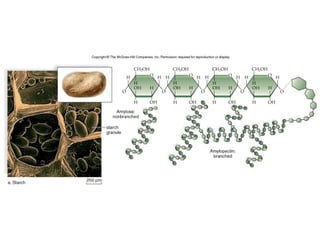
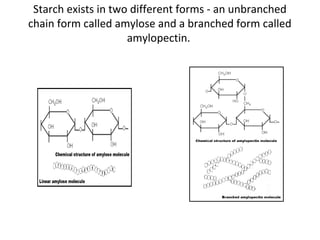
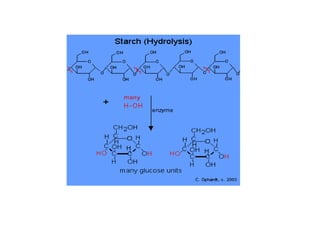
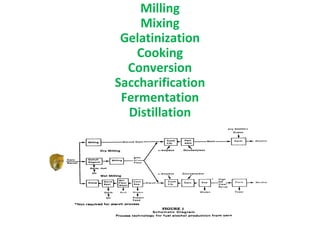
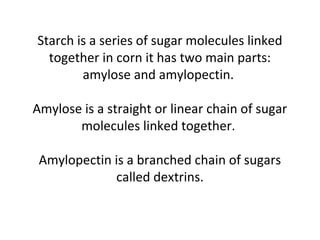- Corn kernels are composed mainly of endosperm, which contains starch, and germ, which contains oil. Starch is made up of amylose and amylopectin chains of glucose molecules.
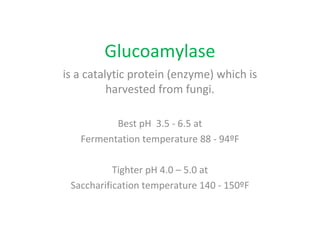
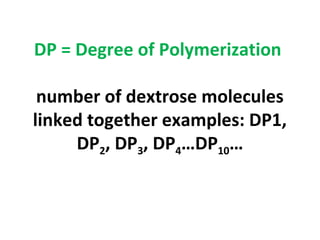
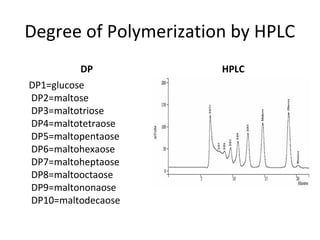
- Alpha-amylase enzymes break the starch down into dextrins and oligosaccharides during cooking and liquefaction. Glucoamylase then breaks down the dextrins and oligosaccharides into individual glucose molecules during saccharification.
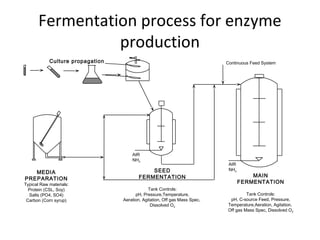
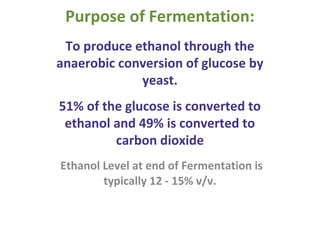
- Yeast ferments the glucose from saccharification into ethanol and carbon dioxide during fermentation, producing the main components of distilled alcohol. The process involves several steps to break down the corn starch into fermentable sugars and then into ethanol.