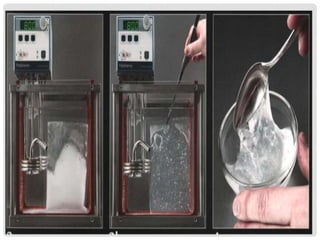
The document discusses the importance of cereals and starches as key food crops, noting that rice, wheat, and corn are the most cultivated. It outlines the sources and classifications of starch, explaining its properties, reactions during cooking, and functional uses in food. Additionally, it provides details on various types of pasta and their preparation methods.