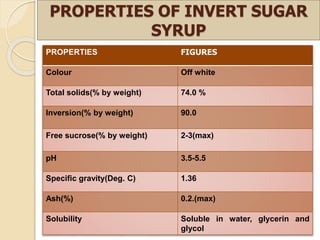
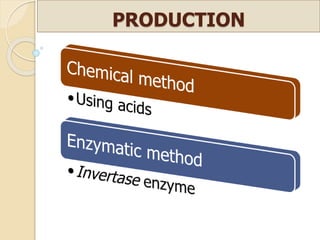
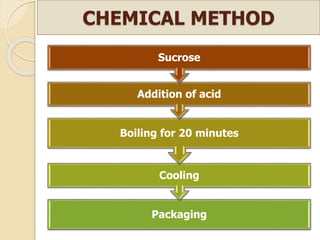
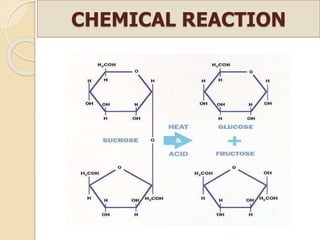
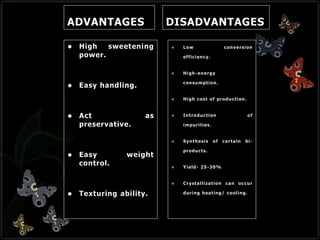
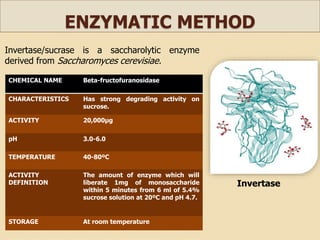
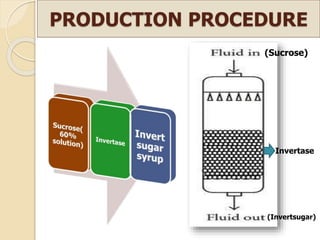
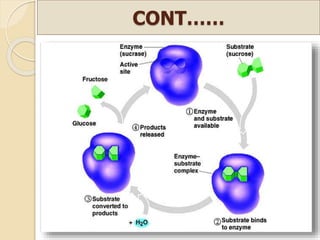
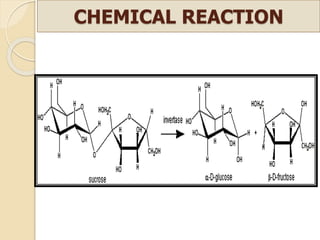
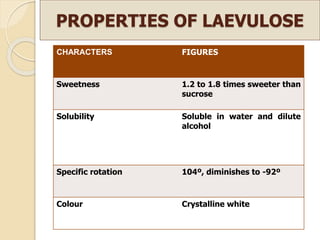
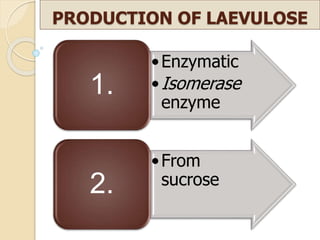
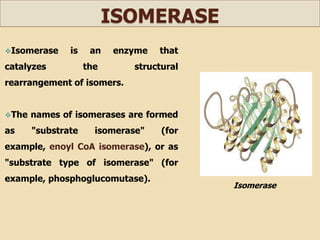
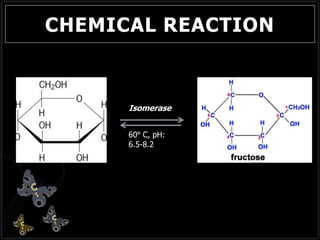
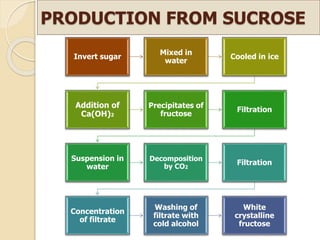
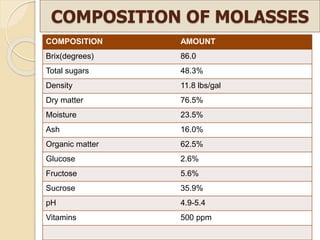
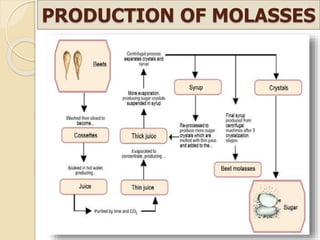
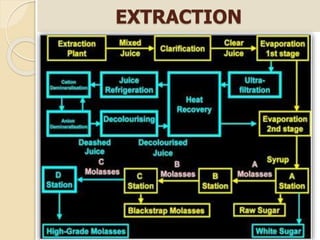
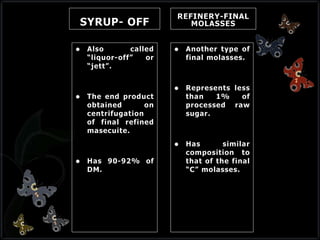
This document discusses invert sugar syrups, their production, and properties. Invert sugar syrup is a mixture of glucose and fructose obtained by splitting sucrose. It has a lower water activity than sucrose, providing better preserving qualities. Invert sugar can be produced through either a chemical method using acids or an enzymatic method using invertase, with the enzymatic method having advantages like higher yield and purity. The document also discusses laevulose/fructose production from sucrose using isomerase enzyme, and types and production of molasses as a viscous by-product of sugar cane or beet processing.