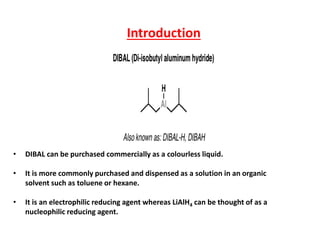
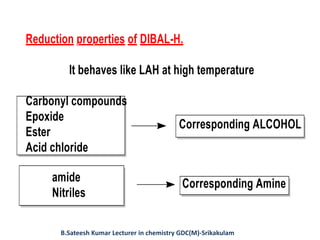
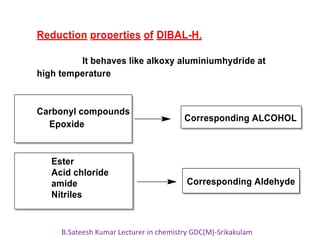
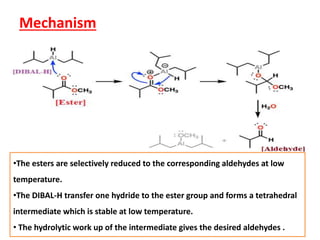
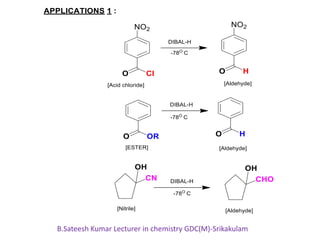
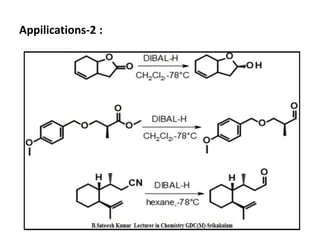
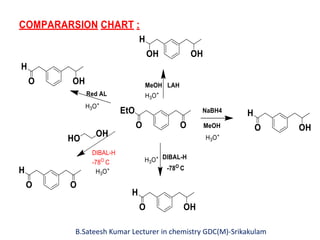
DIBAL-H is a commercially available selective reducing agent that can reduce esters and nitriles to the corresponding aldehydes. It is prepared by heating triisobutylaluminum, which induces beta hydride elimination to form DIBAL-H and isobutene. DIBAL-H selectively reduces esters to aldehydes at low temperatures through a tetrahedral intermediate. Hydrolytic workup of this intermediate then yields the desired aldehyde products. The document provides an introduction to DIBAL-H including its preparation, applications in organic synthesis, and how it differs from other reducing agents like LiAlH4.


![Preparation
Preparation of DIBAL by heating of tri isobutyl aluminium
(itself a dimer) to induce beta hydride elimination:
Equation:
(iso-Bu3Al)3 → (i-Bu2AlH)2 + 2 (CH3)2C=CH2
[tri isobutyl aluminium] [DIBAL-H] [Isobutene]](https://image.slidesharecdn.com/imortanceofdibal-h-200519050631/85/Imortance-of-DIBAL-H-5-320.jpg)

![Thank you
[More videos please subscribe my channel
SATEESH KEYS ]](https://image.slidesharecdn.com/imortanceofdibal-h-200519050631/85/Imortance-of-DIBAL-H-13-320.jpg)