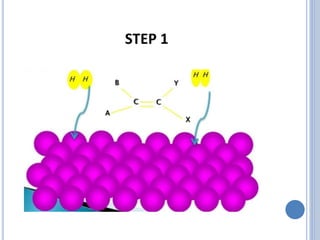
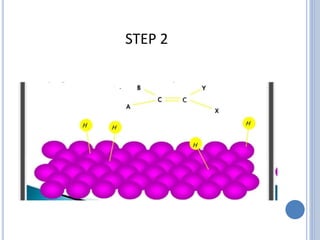
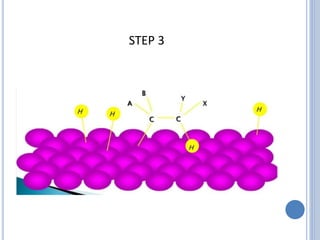
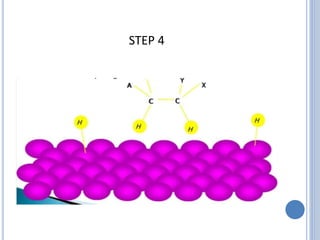
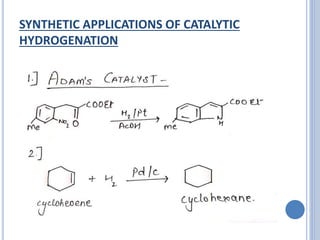
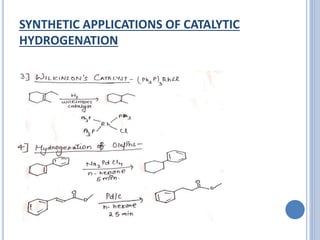
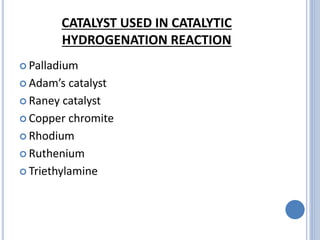
The document discusses catalytic hydrogenation, detailing its mechanism, advantages, and applications, particularly in organic chemistry. It highlights the role of catalysts such as nickel, palladium, and rhodium in facilitating the reaction, as well as contrasting heterogeneous and homogeneous catalytic processes. Various industrial applications, including the Monsanto acetic acid process and the Wacker process, are also mentioned, along with specific steps involved in the reaction mechanism.





![HOMOGENEOUS CATALYTIC
HYDROGENATION
The rhodium complex used is (Ph3P)3RhCl called as
Wilkinson’s catalyst [tris(triphenylphosphine)chlororhodium].
Wilkinson’s catalyst has an advantage that where olefins and
acetylenes get reduced, other common group like C=O, C≡N,
NO2 remain unaffected. Thus selective reduction can be
carried out.
Monsanto acetic acid process:- Monsanto developed the
rhodium catalyzed process for the carbonylation of methanol
to produce acetic acid.
Wacker process:- this is one of the earliest industrial
processes developed in Germany for the conversion of
ethylene into acetaldehyde. Wacker process is more complex
than other catalytic processes.
Hydroformylation:- The reaction of an alkene with carbon
monoxide and hydrogen, catalyzed by cobalt or rhodium salts
to form an aldehyde.](https://image.slidesharecdn.com/aish-200317031809/85/Hydrogenation-catalytic-hydrogenation-8-320.jpg)