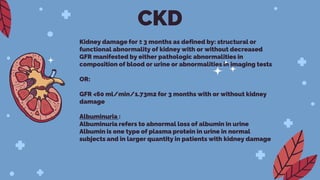
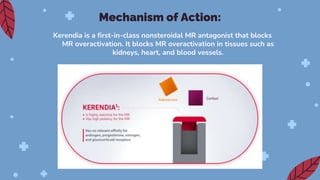
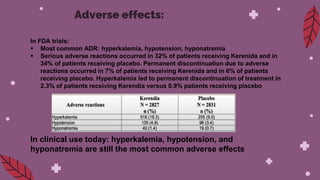
Kerendia is a new prescription medication used to treat chronic kidney disease associated with type 2 diabetes. It works by blocking mineralocorticoid receptors to reduce worsening of kidney function, kidney failure, cardiovascular death, heart attack, and hospitalization for heart failure. The drug was shown in clinical trials to significantly slow kidney disease progression and reduce cardiovascular risks. It is dosed based on serum potassium levels and kidney function, and common side effects include hyperkalemia, hypotension, and hyponatremia. Careful monitoring of potassium and dose adjustments may be needed.