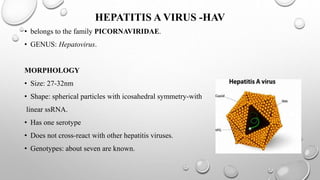
The document discusses several hepatitis viruses including types A, D, E, and G. It provides details on the classification, morphology, transmission, epidemiology, clinical manifestation, laboratory diagnosis, treatment and prevention of each virus. Hepatitis A virus causes infectious hepatitis and is transmitted via the fecal-oral route. Hepatitis D virus is defective and requires hepatitis B virus for replication. It causes more severe disease than hepatitis B alone. Hepatitis E virus causes enterically transmitted hepatitis epidemics in certain regions. Hepatitis G virus does not appear to cause hepatitis but may co-infect with other viruses like HIV.