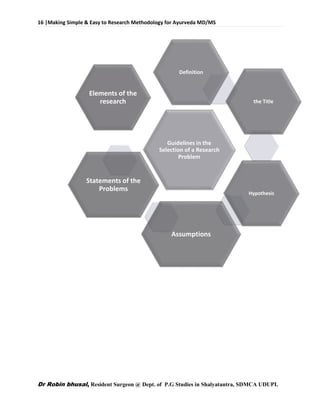
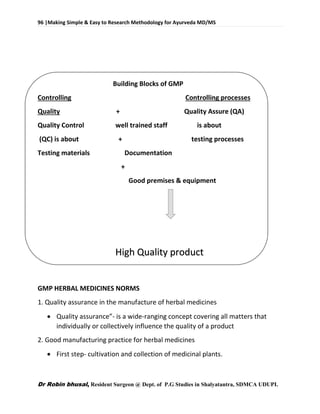
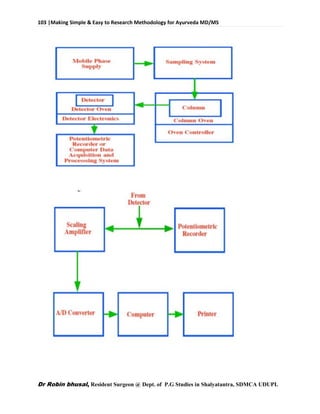
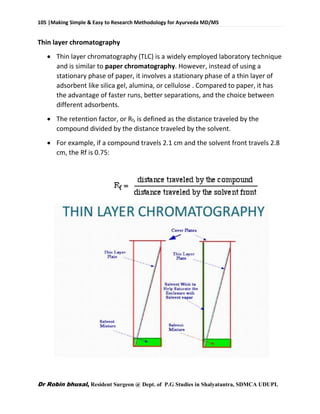
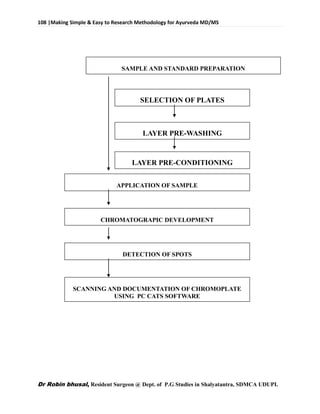
This document provides an overview of research methodology for Ayurveda. It defines key terms like research and anusandhan. Research is described as a systematic and organized process to find answers to questions, while anusandhan refers to following appropriate knowledge to understand cause-effect relationships.
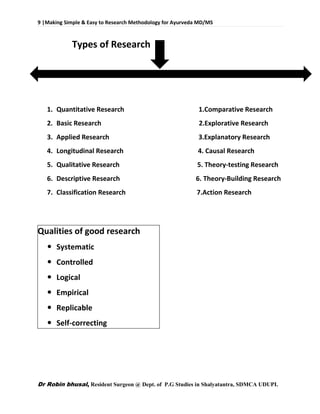
The document outlines different types of research like quantitative, qualitative, descriptive, and more. It also discusses the need for research in Ayurveda to revalidate classics, develop new treatments, and standardize clinical procedures and drugs. Specific areas that could benefit from research are mentioned, such as conceptualizing terms, analyzing relationships, and validating controversial therapies.




















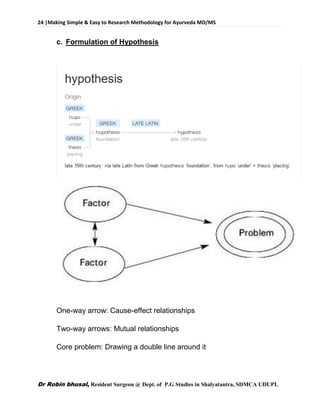
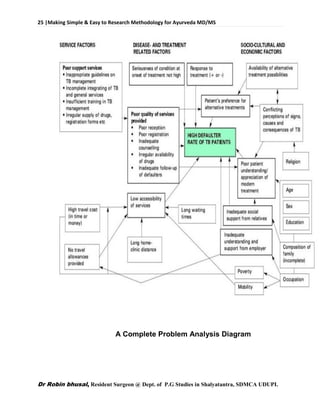



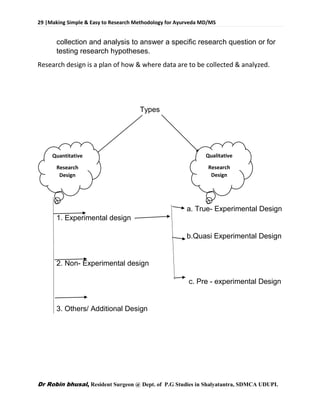
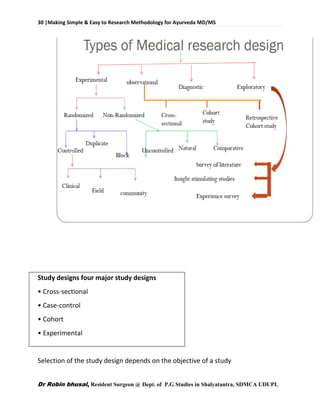


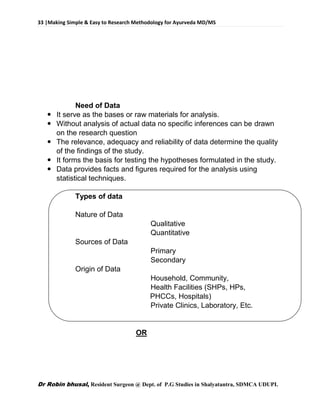








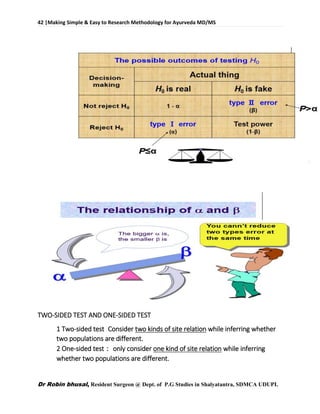
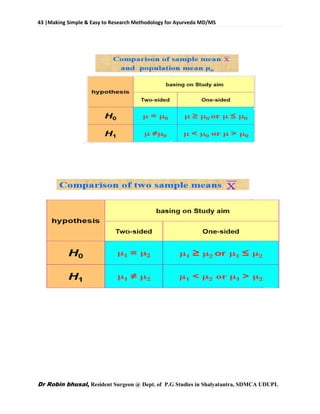
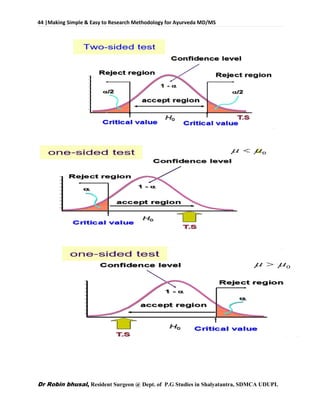

















![62 |Making Simple & Easy to Research Methodology for Ayurveda MD/MS
Dr Robin bhusal, Resident Surgeon @ Dept. of P.G Studies in Shalyatantra, SDMCA UDUPI.
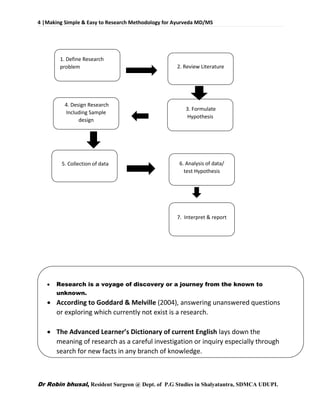
Organization of a scientific paper
The most common is the IMRAD If a number of methods were used to
achieve directly related results
M + R = Experimental section
The results are so complex that they need to be immediately discussed:
R + D = Results and Discussion section
IMRAD (Introduction, Methods, Research [and] Discussion)
• is a mnemonic for a common format used for academic ['scientific'] research
papers. While used primarily in the hard sciences, like physics and biology, it is
also widely used in the social and behavioral sciences. The IMRAD format is also
known as the APA format, as the American Psychological Association uses the
IMRAD headings in its APA style sheet. IMRAD is simply a more 'defined' version
of the "IBC" [Introduction, Body, and Conclusion] format used for all academic
writing.
IMRAD format slowly progressed in the latter half of the 19 th century.
Essential Parts of a scientific paper
Title: Describe concisely the core contents of the paper
Contact info
Abstract, keywords : Summarize the major elements of the
Body of paper:
Introduction: : provide context & rationale for the study
Materials : Describe the experimental design so it is reproducible
Methods: Describe the experimental procedures
Results : Summarize the findings without interpretation
Discussion: Interpret the findings of the study
Acknowledgements: Give credit to those who helped](https://image.slidesharecdn.com/researchmethodologybydrrobinbhusalforayurvedamd-160603172758/85/Research-methodology-for-ayurveda-MD-MS-62-320.jpg)













![76 |Making Simple & Easy to Research Methodology for Ayurveda MD/MS
Dr Robin bhusal, Resident Surgeon @ Dept. of P.G Studies in Shalyatantra, SDMCA UDUPI.
Common data mining tasks
1. Classification [Predictive Tasks]
2. Clustering [Descriptive Tasks]
3. Association Rule Discovery [Descriptive Tasks]
4. Sequential Pattern Discovery [Descriptive Tasks]
5. Regression [Predictive Tasks]
6. Deviation Detection [Predictive Tasks]
1. Classification
It can be used to built up idea of type of patients, diseases, or features of
patients or diagnosis.
2. Clustering
It is useful to identify different information because it co-relates with
other examples so you can see where the similarities and ranges agrees.
3. Association Rule Discovery
It is used to make a simple correlation between two or more items, often
of the same type of identify patterns.
4. Sequential Pattern Discovery
Often used over longer-term data, sequential patterns are a useful method
for identifying trends or regular occurrences of similar events.
5. Regression
To make quantitative predictions of one variable from the values of
another.
6. Deviation Detection
The difference between an observed values and expected value of a
variable or functions.](https://image.slidesharecdn.com/researchmethodologybydrrobinbhusalforayurvedamd-160603172758/85/Research-methodology-for-ayurveda-MD-MS-76-320.jpg)






















































![138 |Making Simple & Easy to Research Methodology for Ayurveda MD/MS
Dr Robin bhusal, Resident Surgeon @ Dept. of P.G Studies in Shalyatantra, SDMCA UDUPI.
SCOPUS
Scopus is a bibliographic
database containing abstracts and citations for academic journal articles. It
covers nearly 22,000 titles from over 5,000 publishers, of which 20,000
are peer-reviewed journals in the scientific, technical, medical, and social
sciences (including arts and humanities).[1]
It is owned by Elsevier and is
available online by subscription. Searches in Scopus also incorporate
searches of patent databases.
Since Elsevier is the owner of Scopus and is also one of the main
international publishers of scientific journals, an independent and
international Scopus Content Selection and Advisory Board was established
to prevent a potential conflict of interest in the choice of journals to be
included in the database and to maintain an open and transparent content
coverage policy, regardless of publisher. The board consists of scientists
and subject librarians.](https://image.slidesharecdn.com/researchmethodologybydrrobinbhusalforayurvedamd-160603172758/85/Research-methodology-for-ayurveda-MD-MS-138-320.jpg)










![150 |Making Simple & Easy to Research Methodology for Ayurveda MD/MS
Dr Robin bhusal, Resident Surgeon @ Dept. of P.G Studies in Shalyatantra, SDMCA UDUPI.
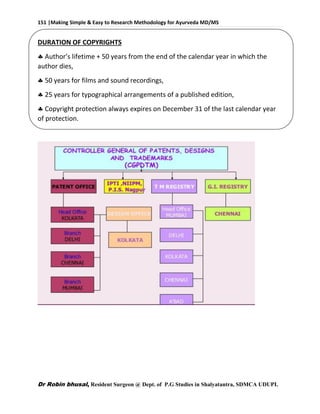
COPYRIGHT
The Indian CopyrightAct, 1957 governs the system of copyrights in India.
[Amended in 1982, 1984, 1992, 1994 & 1999]
Meaning: It is a right which Grants protection to the unique expression of Ideas.
Covered by copyrights
Literary
Films
Dramatic
Musical
Artistic
Sound Recording](https://image.slidesharecdn.com/researchmethodologybydrrobinbhusalforayurvedamd-160603172758/85/Research-methodology-for-ayurveda-MD-MS-150-320.jpg)