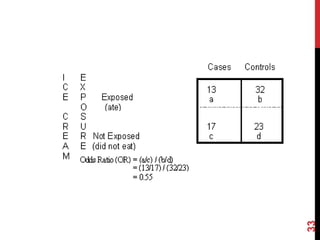
This document discusses different epidemiological research designs including cross-sectional studies, cohort studies, and case-control studies. Cross-sectional studies measure prevalence of disease or risk factors at a single point in time. Cohort studies follow groups over time to measure incidence. Case-control studies identify existing cases and look back to compare exposures between cases and controls. Each design has advantages and limitations for establishing causality and informing public health strategies.