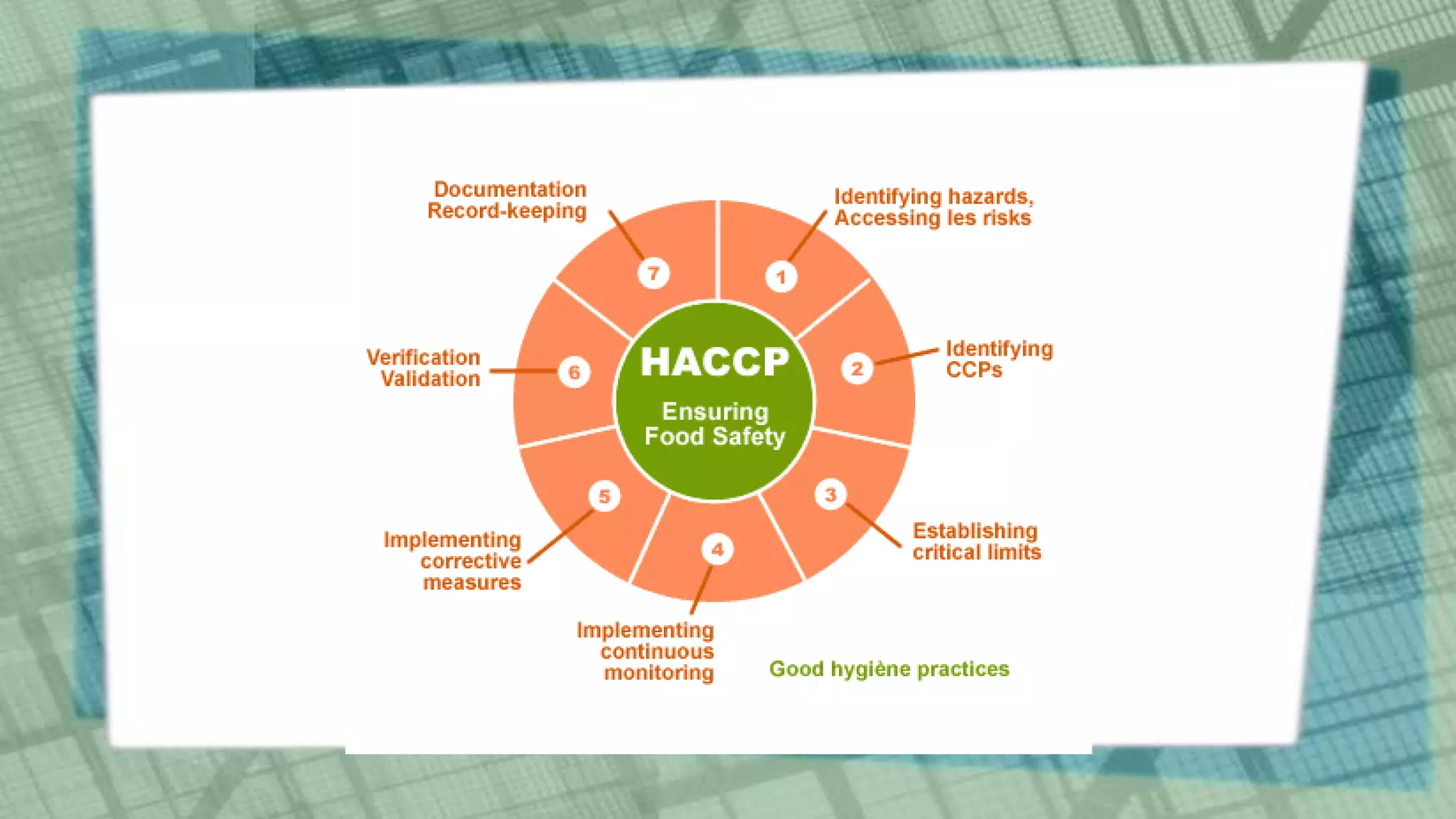
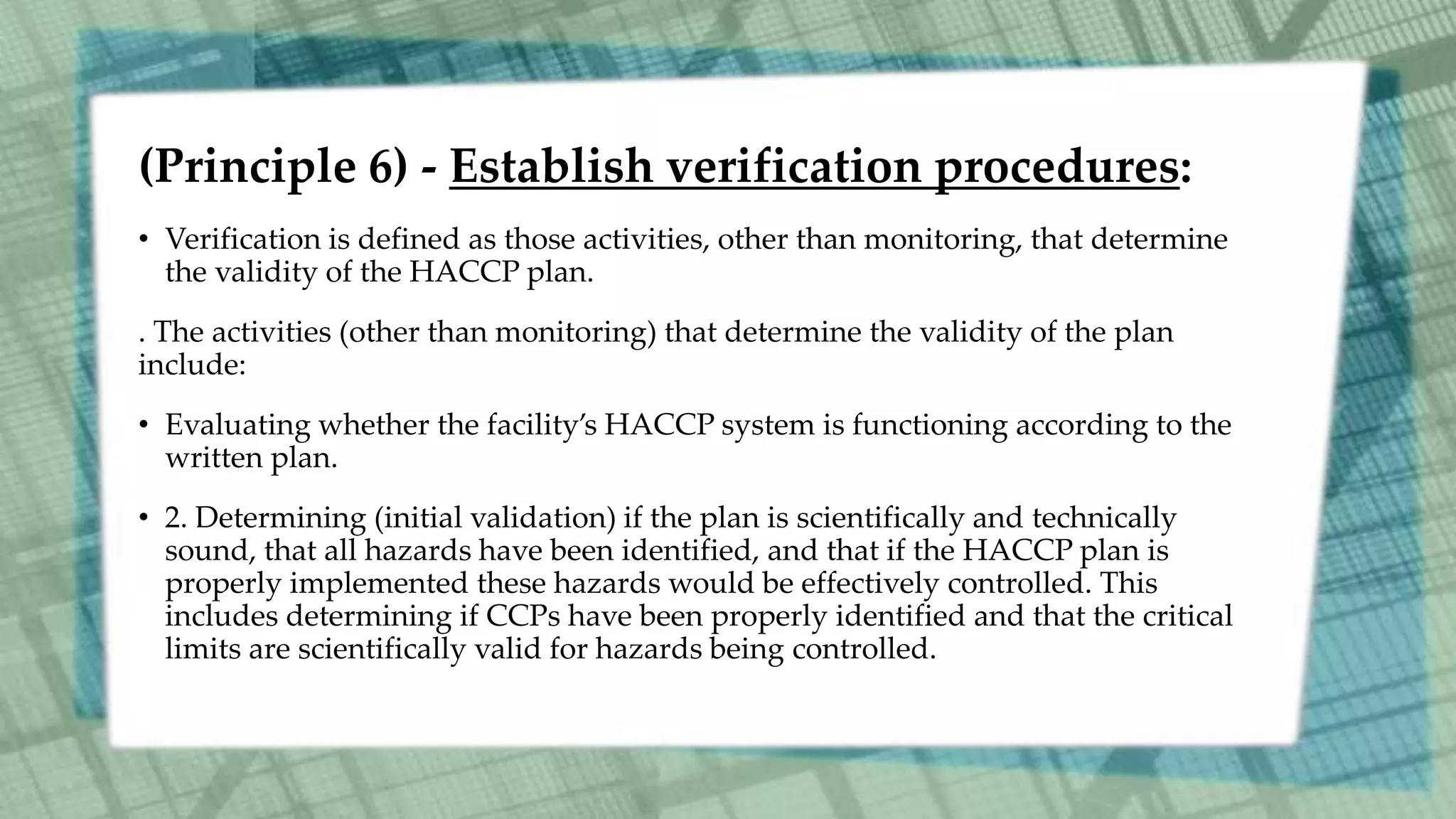
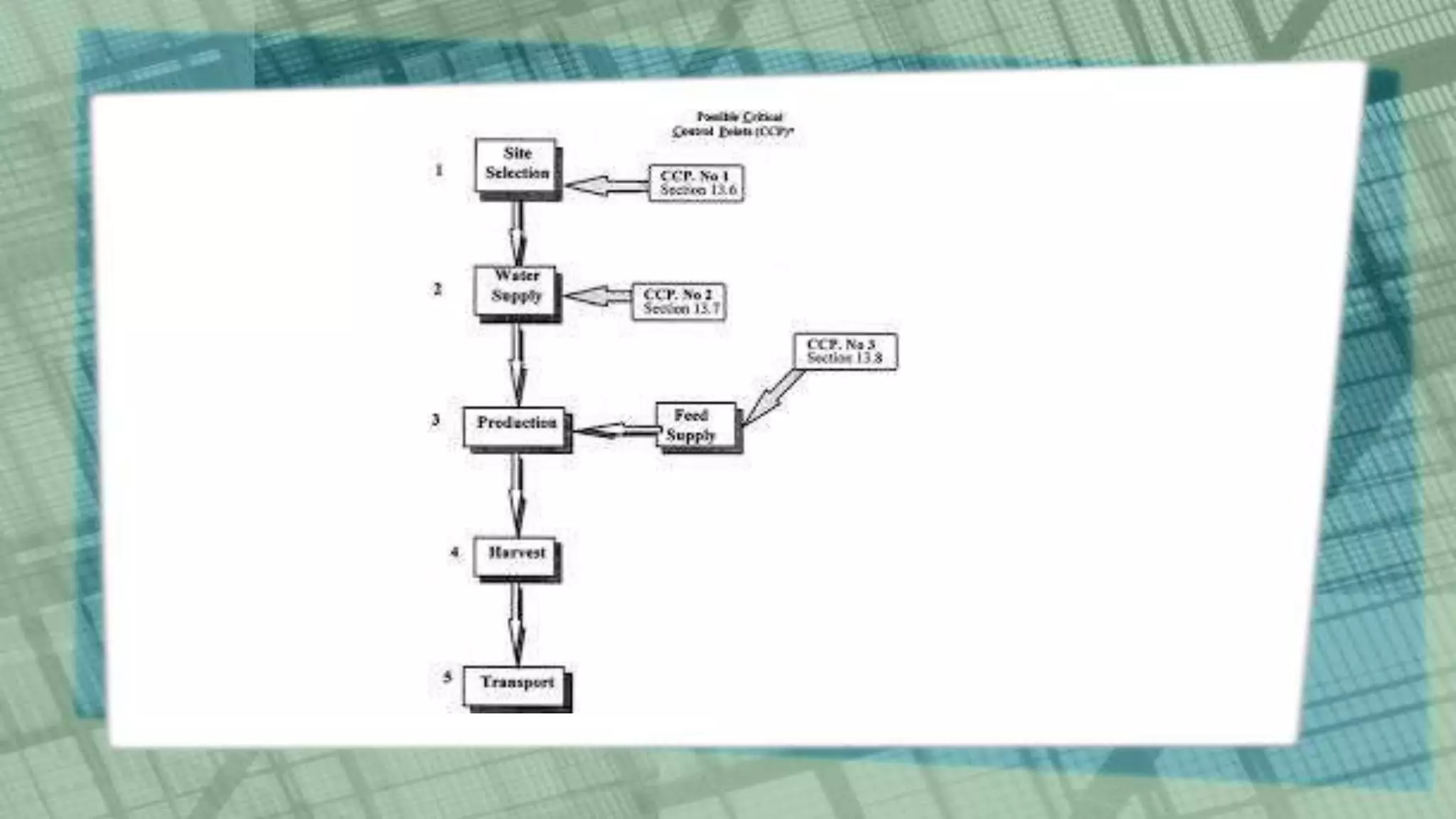
This document discusses Hazard Analysis and Critical Control Points (HACCP) in aquaculture. It defines key HACCP terms like hazard, hazard analysis, critical control point, critical limit, deviation, monitoring, corrective actions, verification, and record keeping. It explains the seven principles of HACCP: conduct hazard analysis, determine critical control points, establish critical limits, establish monitoring procedures, establish corrective actions, establish verification procedures, and establish record keeping procedures. For each principle, it provides details on how to implement that principle as part of a HACCP plan to ensure food safety is managed effectively in aquaculture operations.