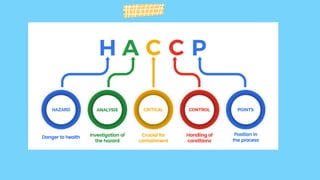
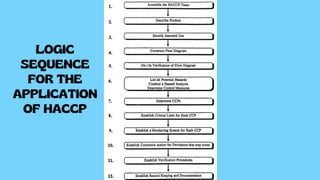
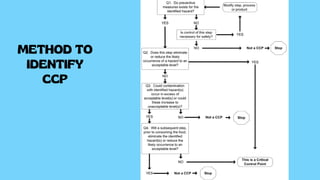
The document provides a comprehensive overview of HACCP (Hazard Analysis and Critical Control Points), a systematic food safety management tool that identifies and controls biological, chemical, and physical hazards throughout the food production process. It details the seven principles of HACCP, including hazard analysis, critical control points, monitoring systems, and corrective actions, emphasizing the importance of documentation and record-keeping. The conclusion highlights HACCP as a preventive measure that enhances food safety and promotes awareness of safety issues among all stakeholders involved.