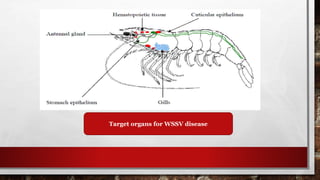
White spot syndrome virus (WSSV) is a highly lethal pathogen affecting penaeid shrimp, causing up to 100% mortality in farms and resulting in significant economic losses. The disease primarily targets juvenile shrimp, and transmission occurs through infected dead shrimp, with symptoms including lethargy, swollen body parts, and visible white spots. Control measures include improving shrimp immunity through specific feed additives and implementing good management practices, but no effective vaccines are currently available.