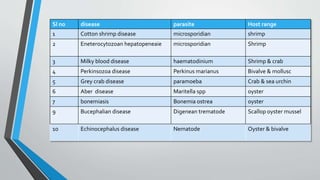
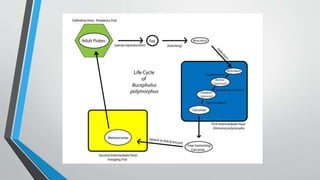
1. This document summarizes 10 parasitic diseases that affect finfish and shellfish: cotton shrimp disease, Hepatopancreatic microsporidiosis, milky blood disease, Perkinsozoa disease, grey crab disease, Aber disease, bonemiasis, Bucephalian disease, Echinocephalus disease, and diseases caused by polychaetes.
2. The diseases are caused by various parasites including microsporidians, Hematodinium, Perkinsus, Paramoeba, Marteilia, and Bonamia that infect important aquaculture species like shrimp, crab, oyster, mussel and more.
3. The diseases cause