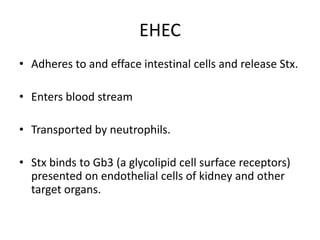
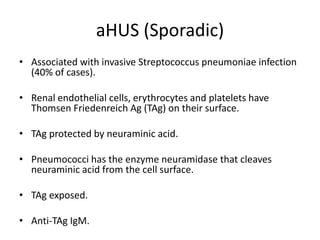
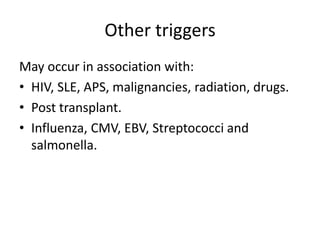
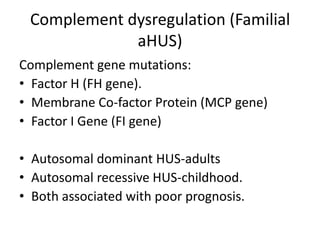
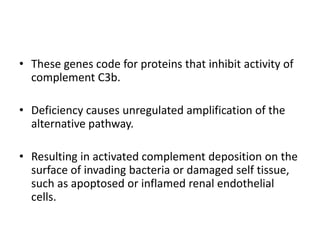
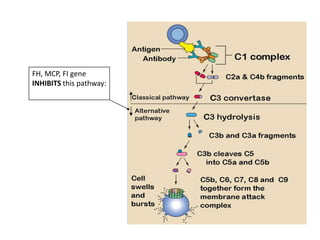
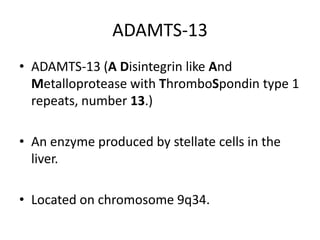
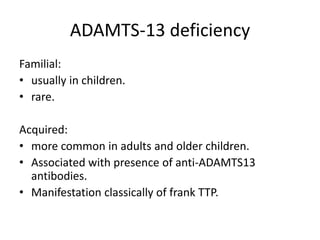
Haemolytic uremic syndrome (HUS) is a clinical syndrome characterized by renal failure, microangiopathic hemolytic anemia, and thrombocytopenia. It is most commonly caused by Shiga toxin-producing bacteria like E. coli O157:H7 through damage to endothelial cells. There are two main categories of HUS - Shiga-toxin associated HUS and non-Shiga-toxin associated HUS. Treatment for HUS focuses on symptomatic relief through dialysis, blood transfusions, and managing complications. The prognosis is generally good, though outcomes can be worse in cases with very high white blood cell counts or persistent kidney damage and failure.