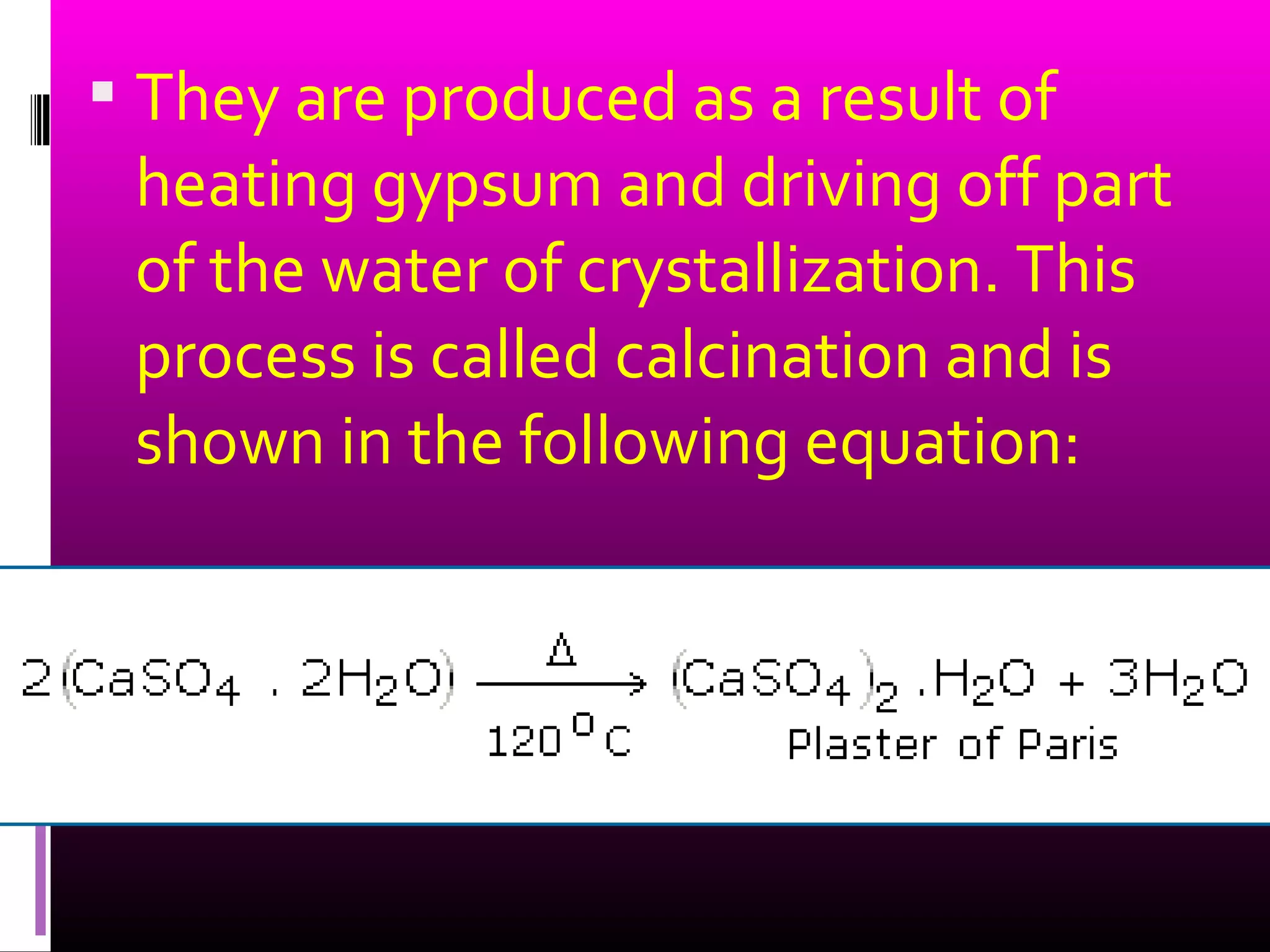
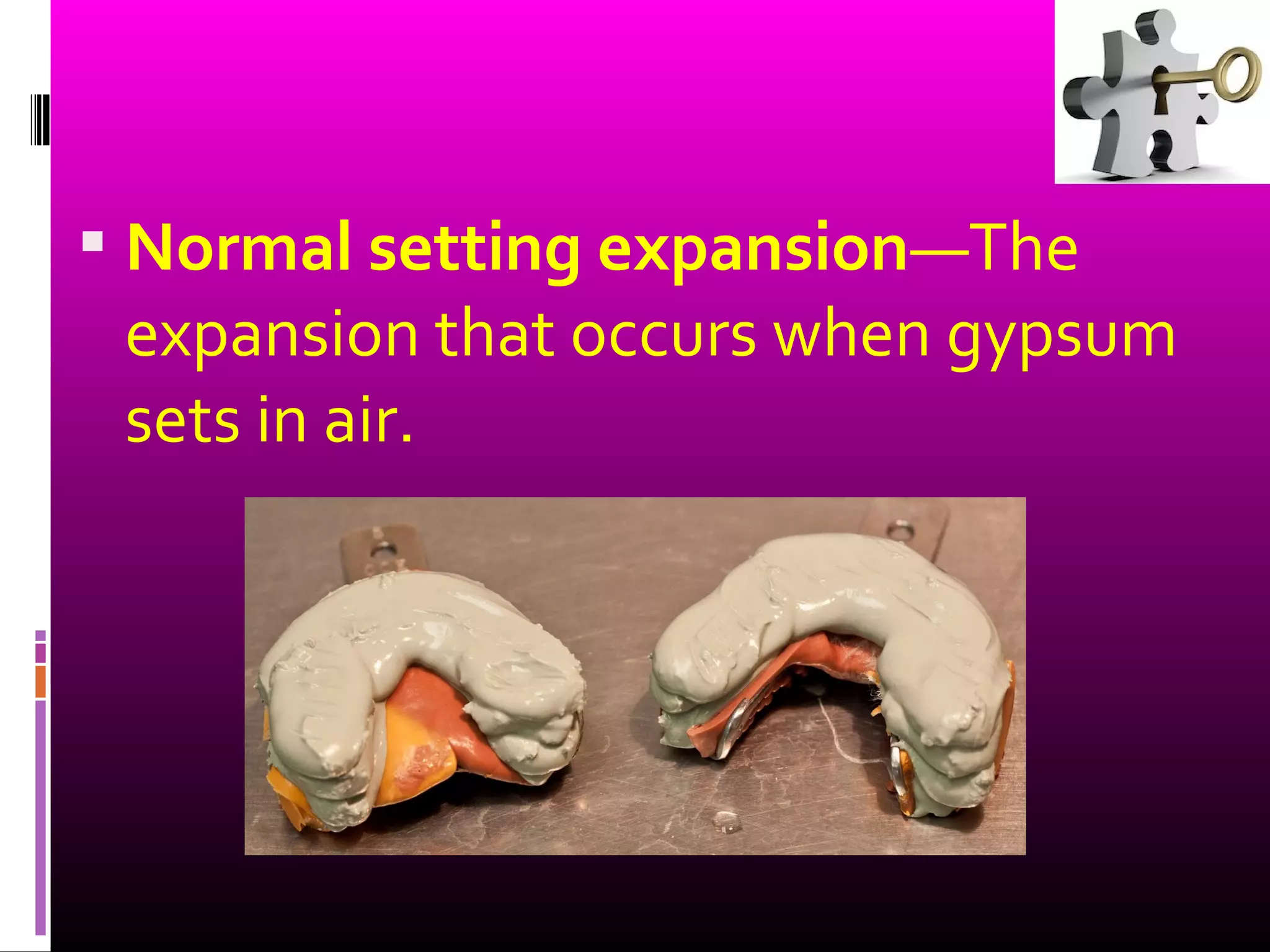
This document discusses different types of gypsum products used in dentistry, including dental plaster, stone, and improved stone. It describes the differences between these products in terms of their crystal structure, density, strength, and setting properties. Dental plaster is the most porous and weakest, while improved stone has the densest and strongest crystals. The document also explains how the setting times and strengths of gypsum products can be modified by adjusting the water-powder ratio or adding chemicals during mixing.