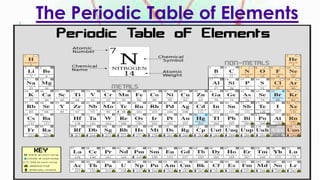
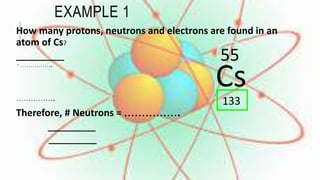
Groups in the periodic table contain elements with the same number of electrons in their outermost shell, giving them similar chemical properties. Periods contain elements with the same number of electron energy levels. The periodic table arranges the 118 known elements and provides information about their properties based on their position and relationships between protons, neutrons, and electrons.