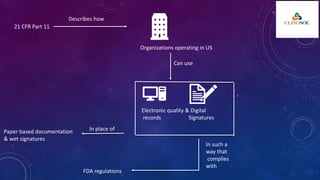
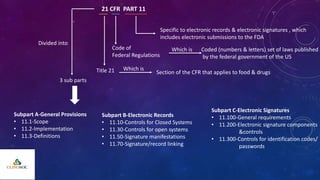
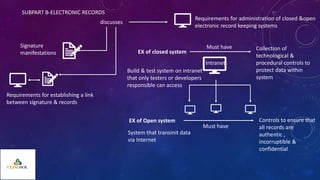
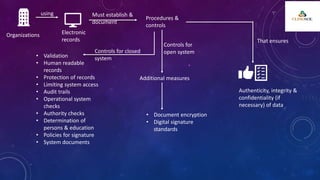
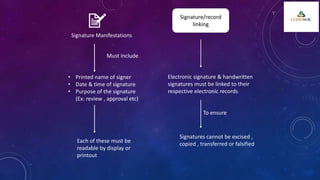
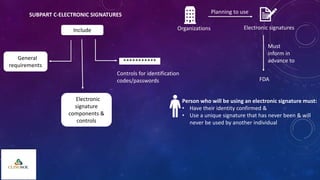
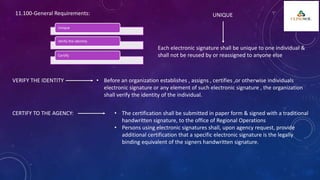
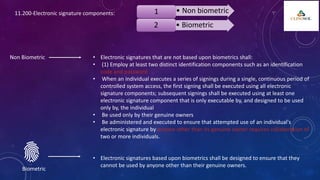
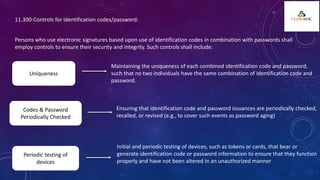
The document outlines 21 CFR Part 11 regulations regarding the use of electronic records and signatures in place of traditional paper documentation in compliance with FDA standards. It details the criteria for electronic records to be considered trustworthy and equivalent to paper records, the requirements for closed and open systems, and specific controls for electronic signatures. Organizations using these electronic methods must maintain strict security measures, ensuring that signatures are unique, verified, and securely linked to their respective records.