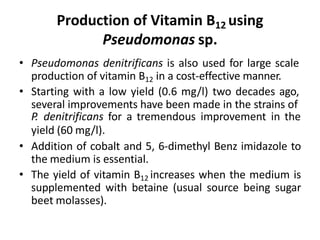
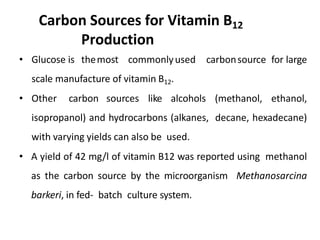
The document discusses the production of L-glutamic acid and vitamin B12 through fermentation. It describes how Dr. Ikeda first isolated glutamic acid from kelp in 1908 and how Dr. Ukada and Dr. Kinoshita later isolated Corynebacterium glutamicum, which enabled the commercial production of monosodium glutamate (MSG). It also discusses the biosynthetic pathways and commercial production methods for both glutamic acid and vitamin B12 using various microorganisms like C. glutamicum and Propionibacterium species. The recovery processes for obtaining the final products are also summarized.
![L-Glutamic acid Production
BTG203A62: BIOTECHNOLOGY VIII
[INDUSTRIAL BIOTECHNOLOGY]](https://image.slidesharecdn.com/l-glutamic-acid-production-230522050311-4e1da974/85/L-Glutamic-acid-Production-pptx-1-320.jpg)