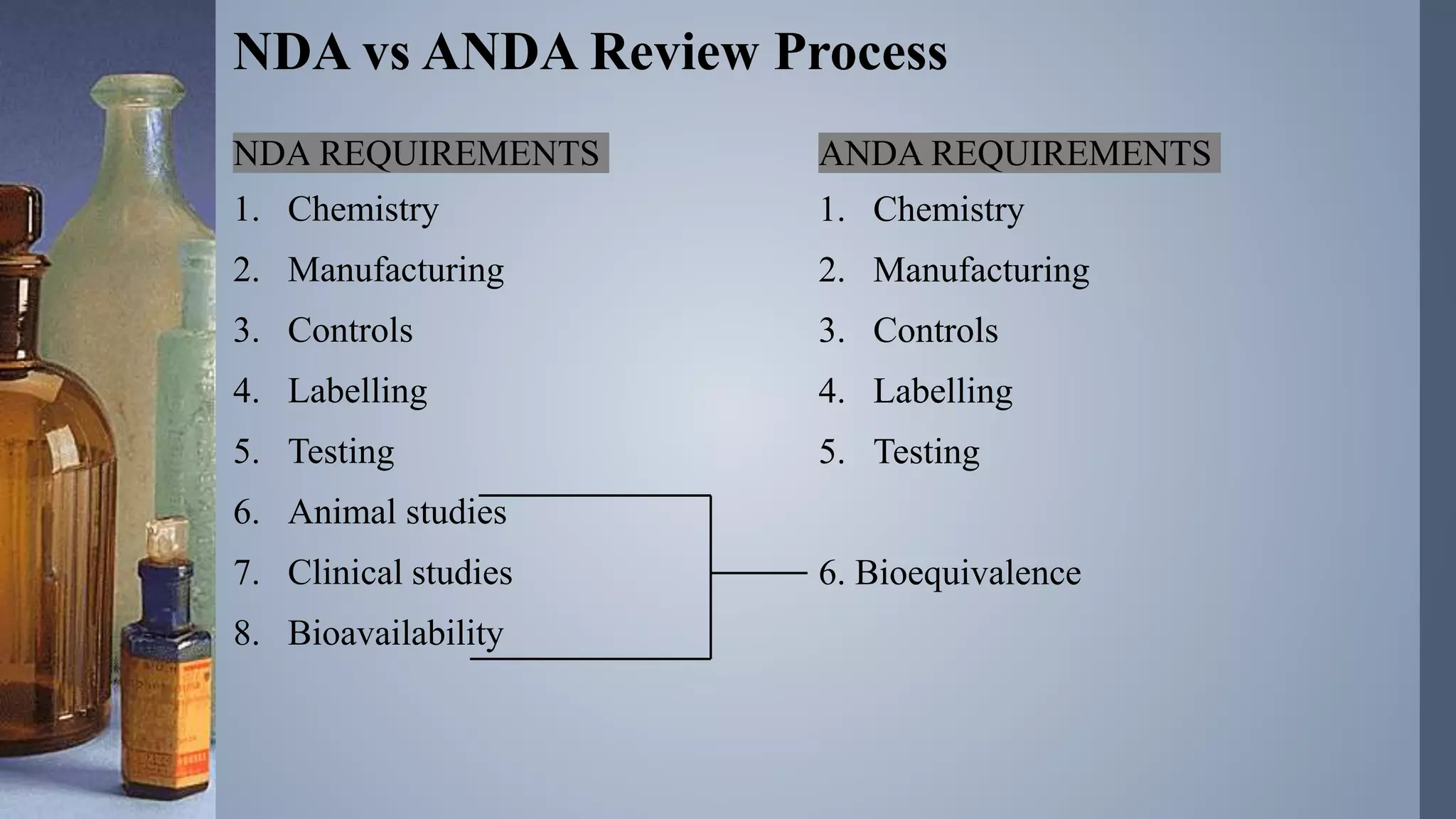
An abbreviated new drug application (ANDA) is a request to the FDA to manufacture and market a generic drug in the U.S., requiring less information than a new drug application as it does not necessitate clinical trials. Generic drugs are similar to brand-name drugs in terms of safety and efficacy, leading to lower costs due to reduced regulatory requirements and increased market competition. The document outlines the ANDA submission process, requirements, and review procedures established to ensure that generics deliver the same clinical benefits as their brand-name counterparts.