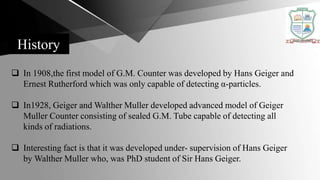
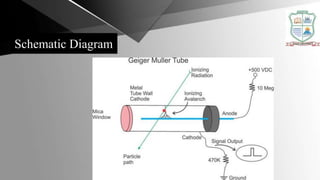
The Geiger-Müller counter is an instrument that detects ionizing radiation such as alpha particles, beta particles, and gamma rays using the ionization effect produced in a Geiger-Müller tube. It consists of a Geiger-Müller tube filled with an inert gas and high voltage that detects radiation by producing a pulse of current. Geiger counters are used to detect radioactive materials in various applications such as mineral prospecting, checking for radiation exposure or leakage, and testing for radioactive contamination of various materials.