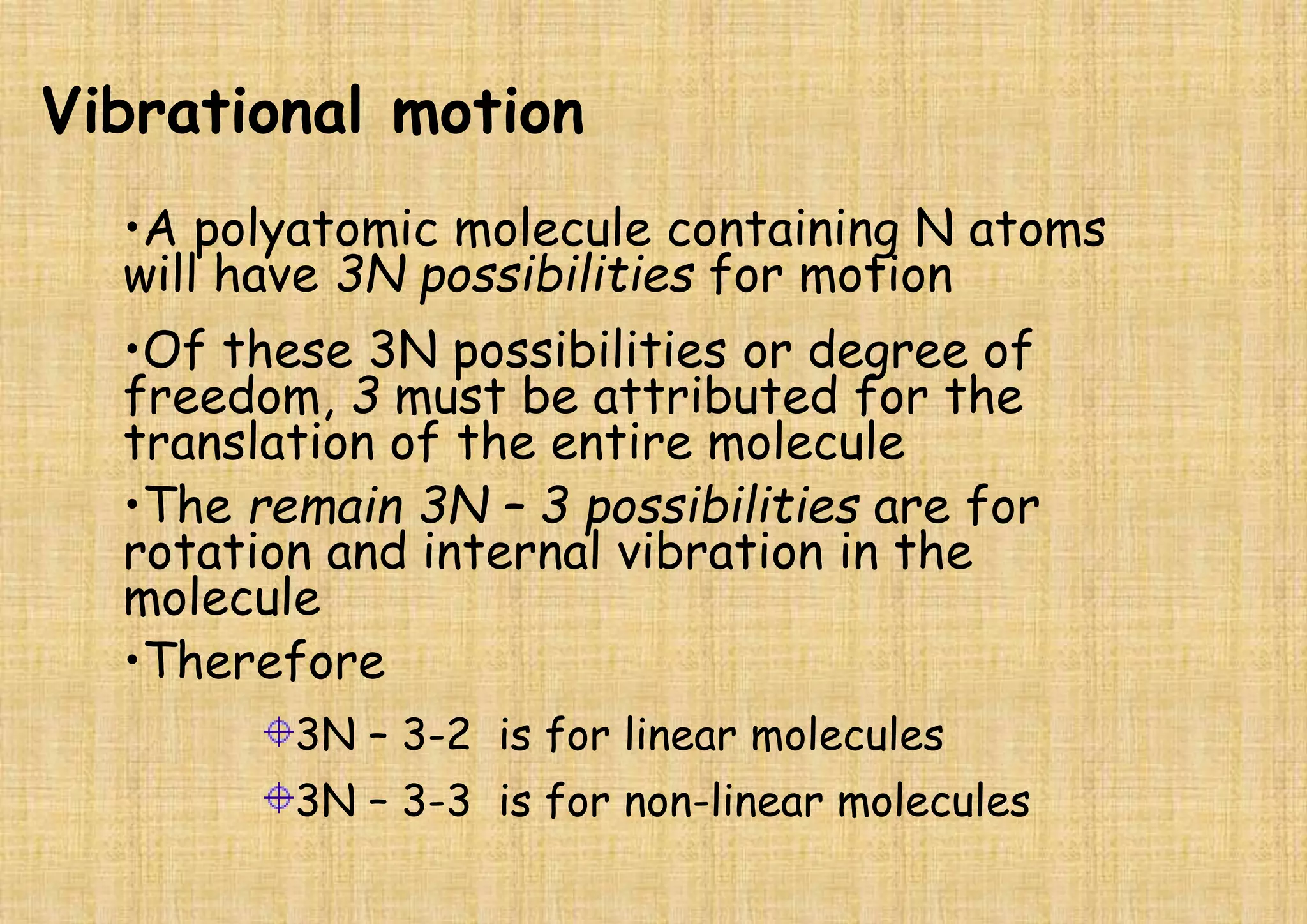
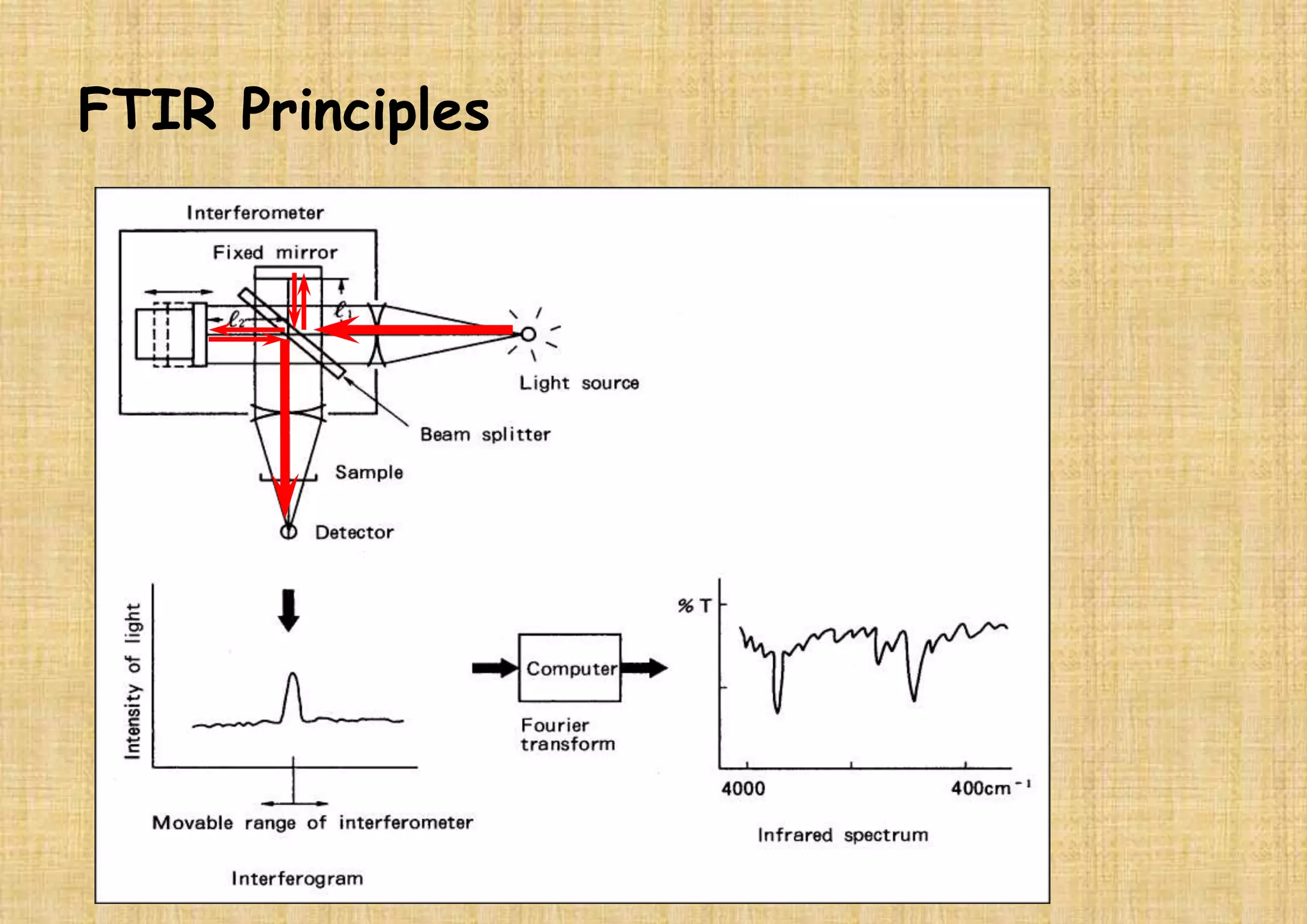
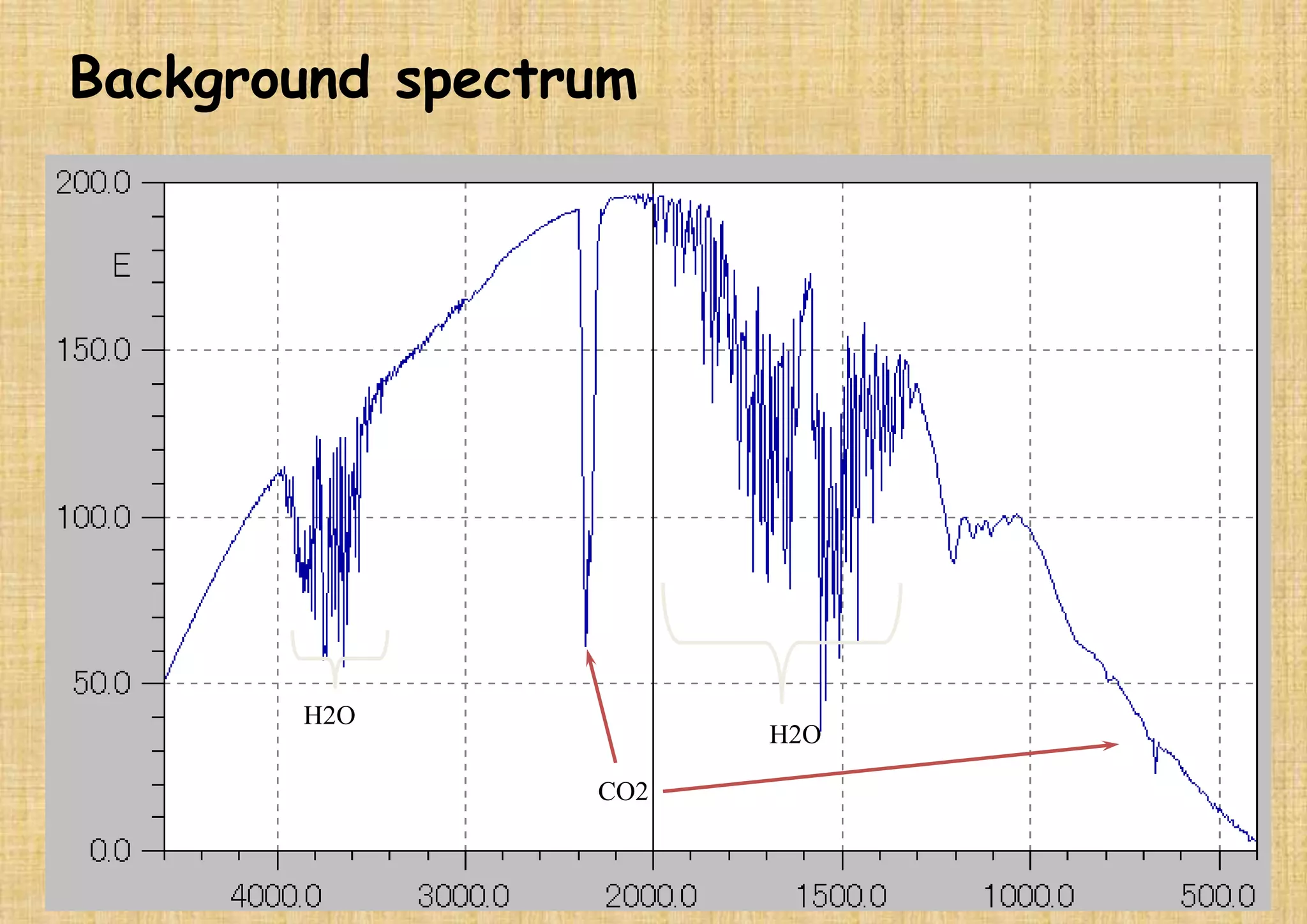
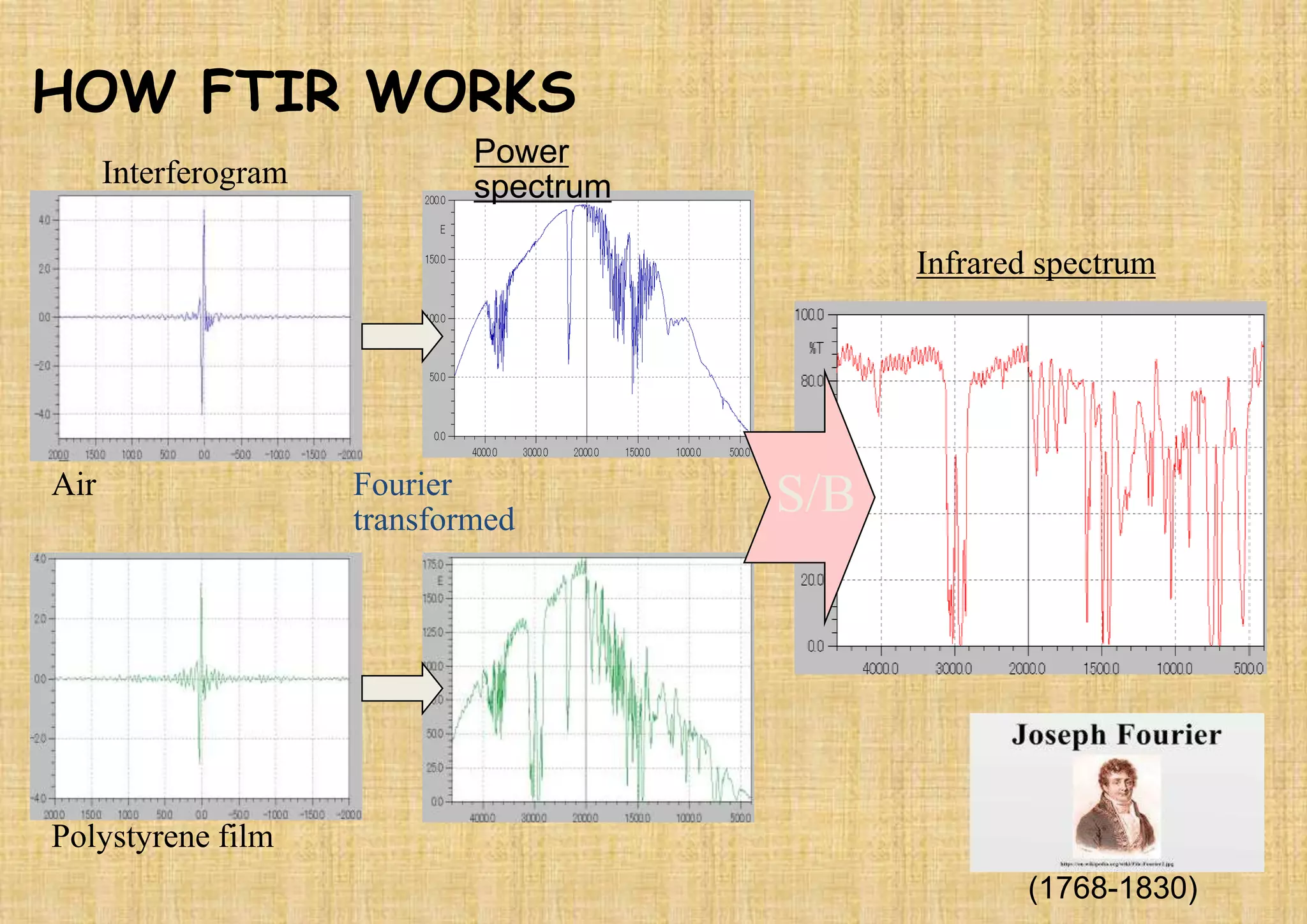
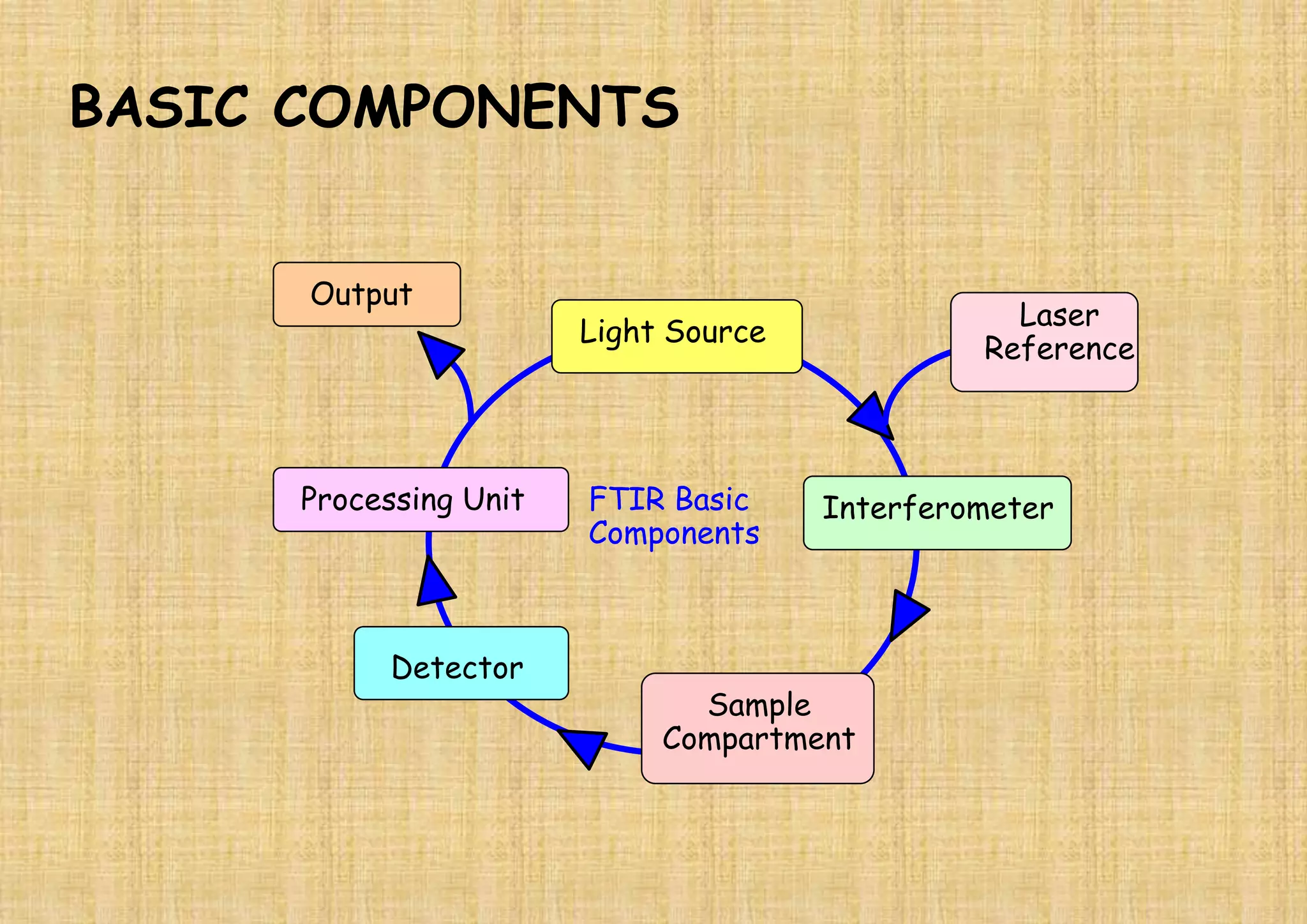
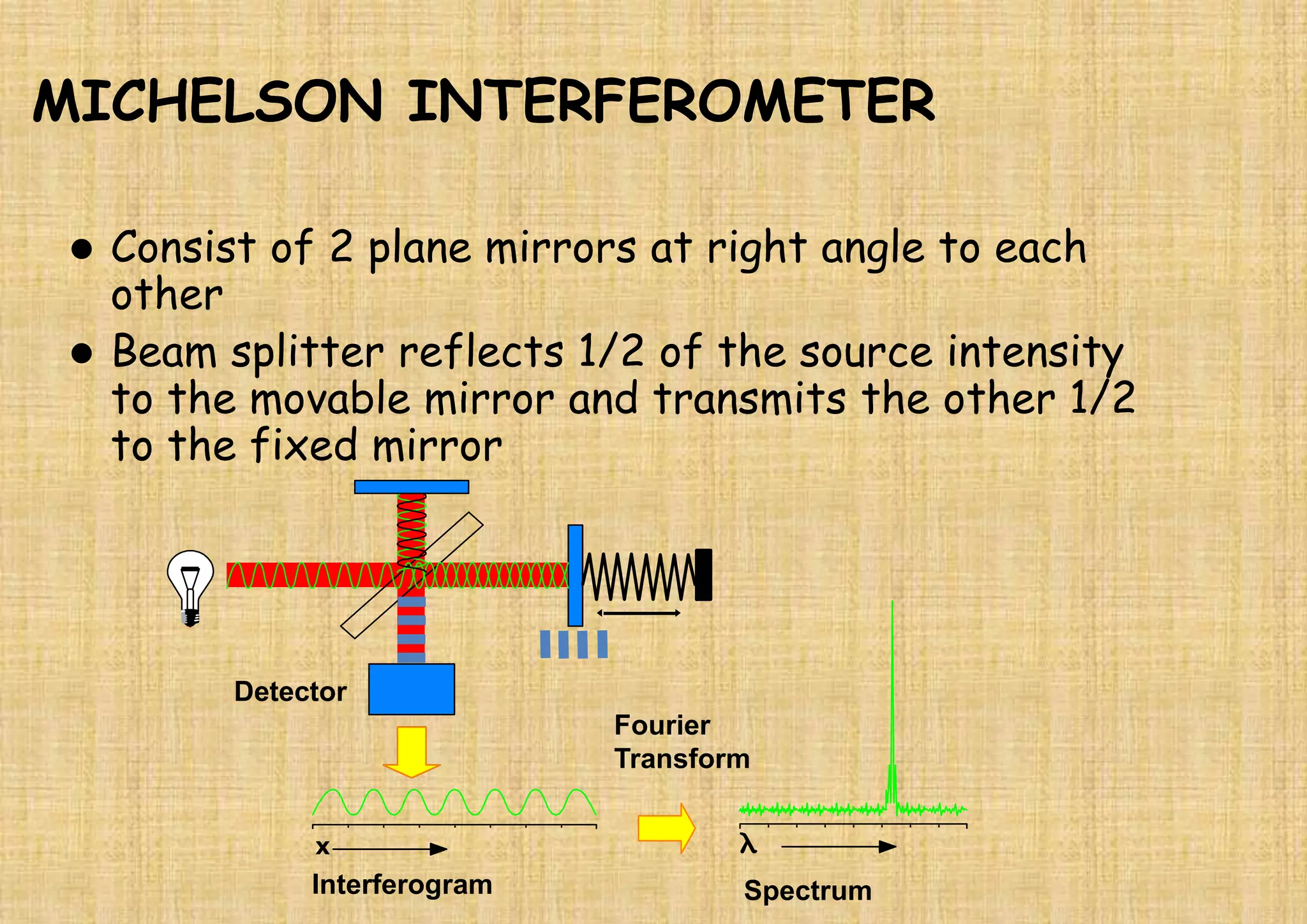
This document provides an overview of Fourier transform infrared spectroscopy (FTIR) theory and instrumentation. It discusses the electromagnetic spectrum and discovery of infrared radiation. It describes the principles of infrared absorption including electronic, vibrational and rotational transitions. The key components of an FTIR instrument are described including the infrared light source, Michelson interferometer, sample compartment, detectors and processing unit. Common infrared window materials and their properties are also summarized.