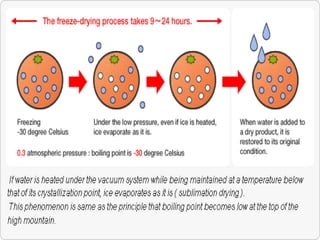
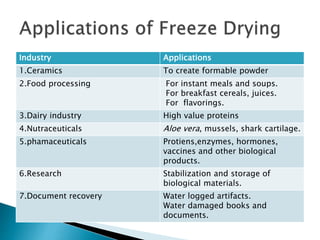
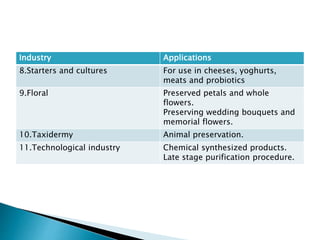
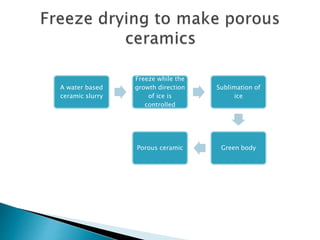
The document discusses the freeze evaporation dehydration process used for preserving materials and enhancing transportation efficiency, which involves four stages: pretreatment, freezing, primary drying, and secondary drying. It highlights the advantages and applications of this technique across various industries, including food processing, pharmaceuticals, and ceramics, while also addressing its limitations such as high costs and potential structural damage. The process is characterized by the sublimation of water and the controlled use of vacuum to optimize drying.