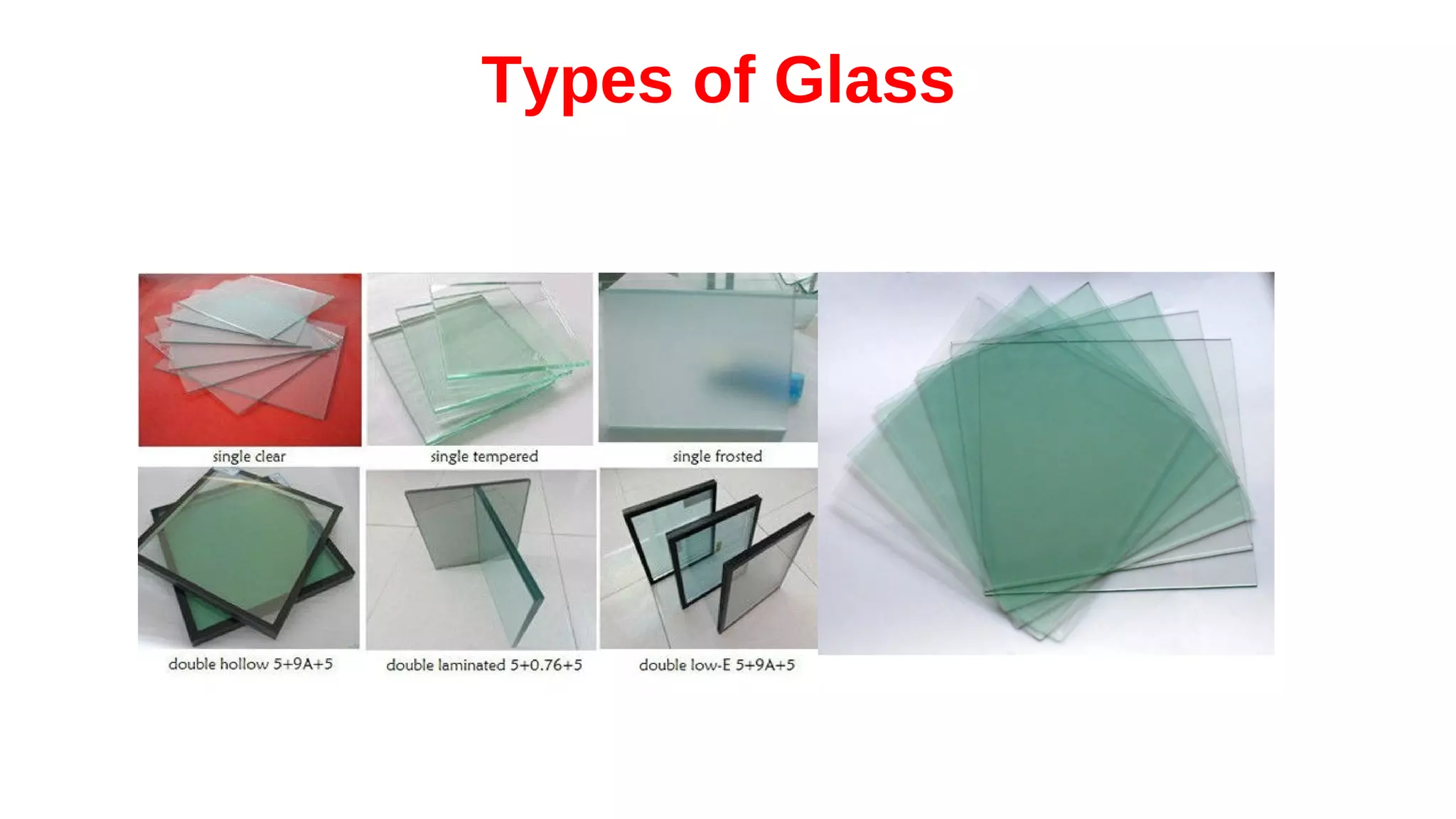
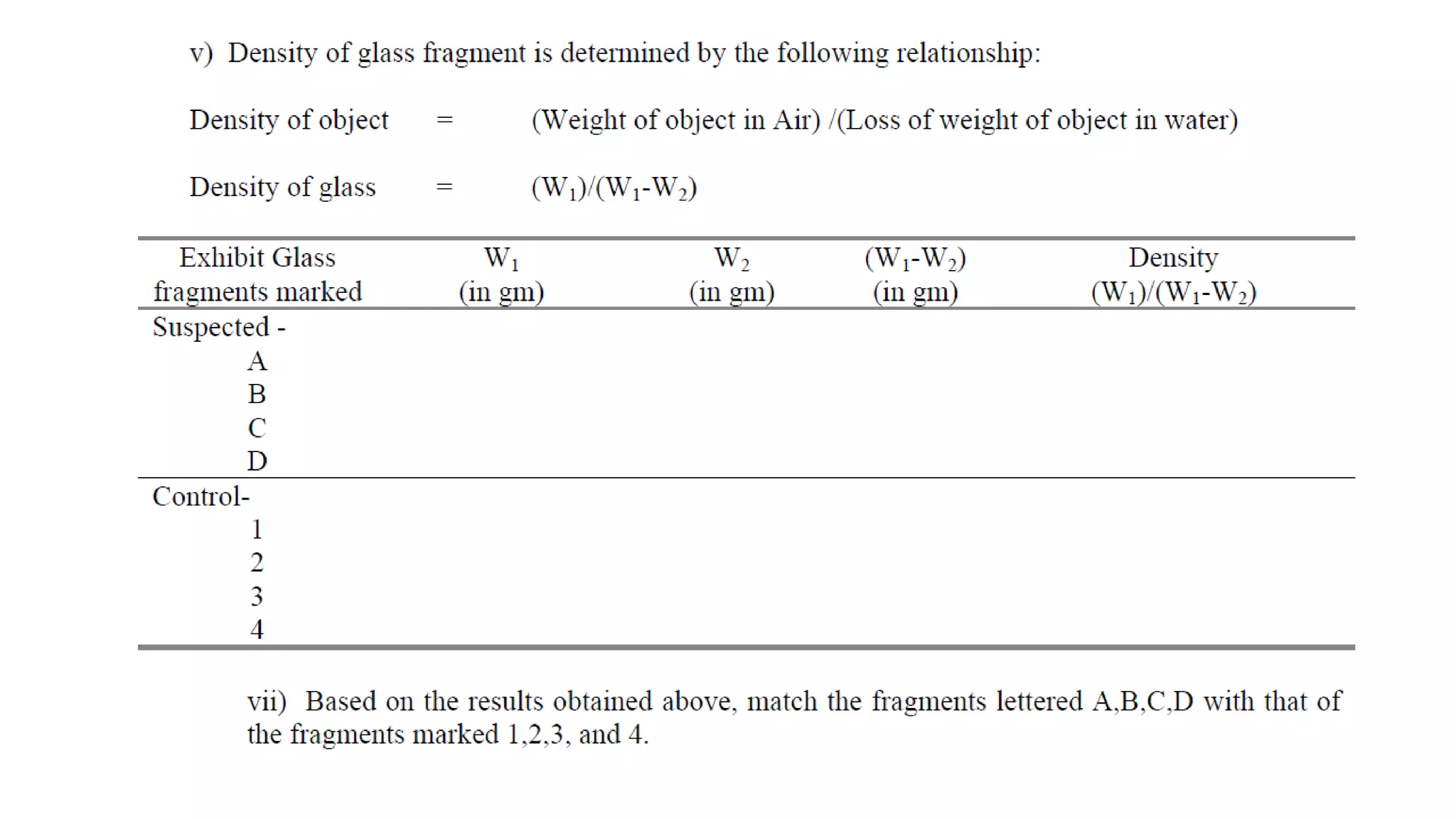
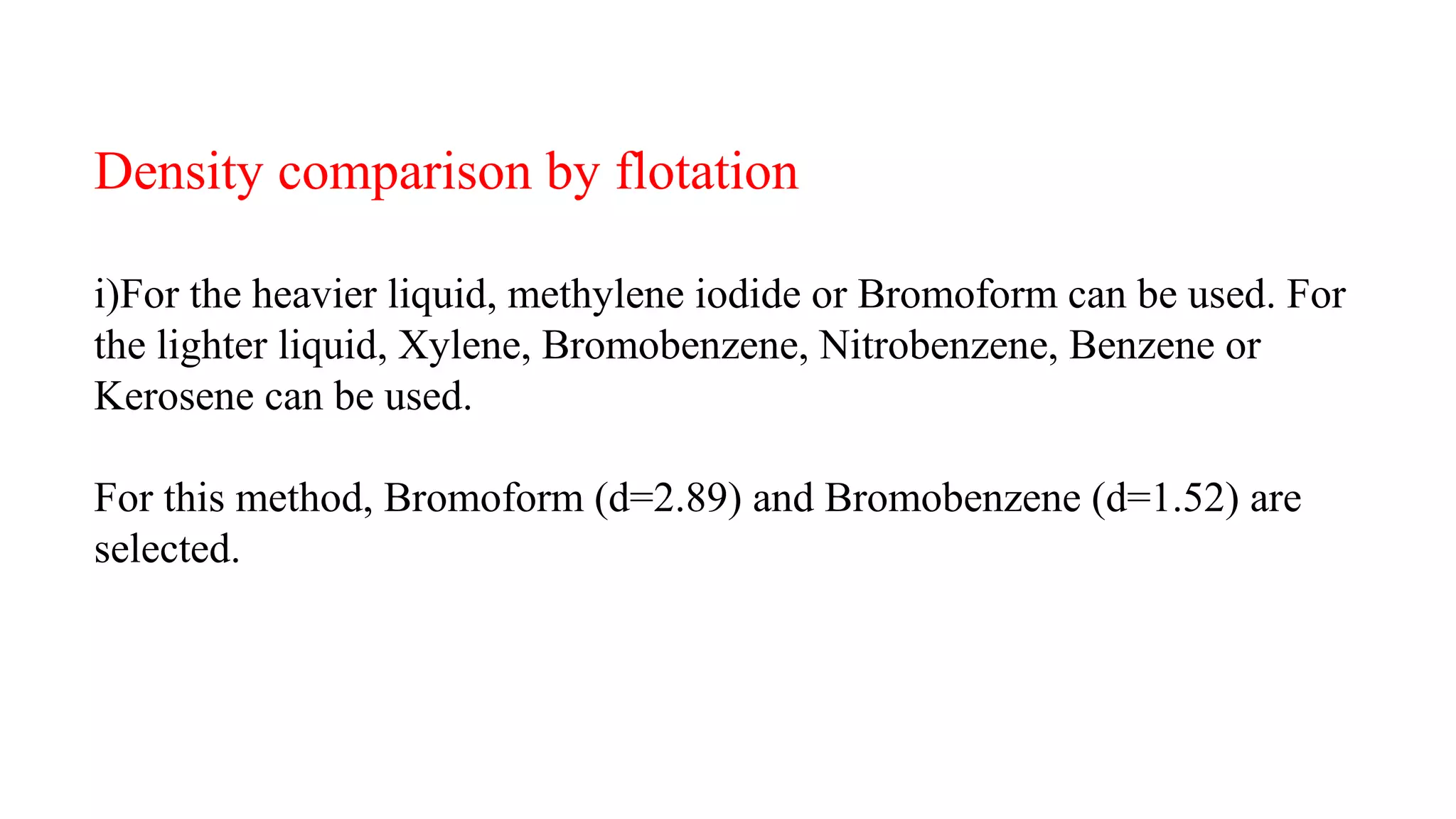
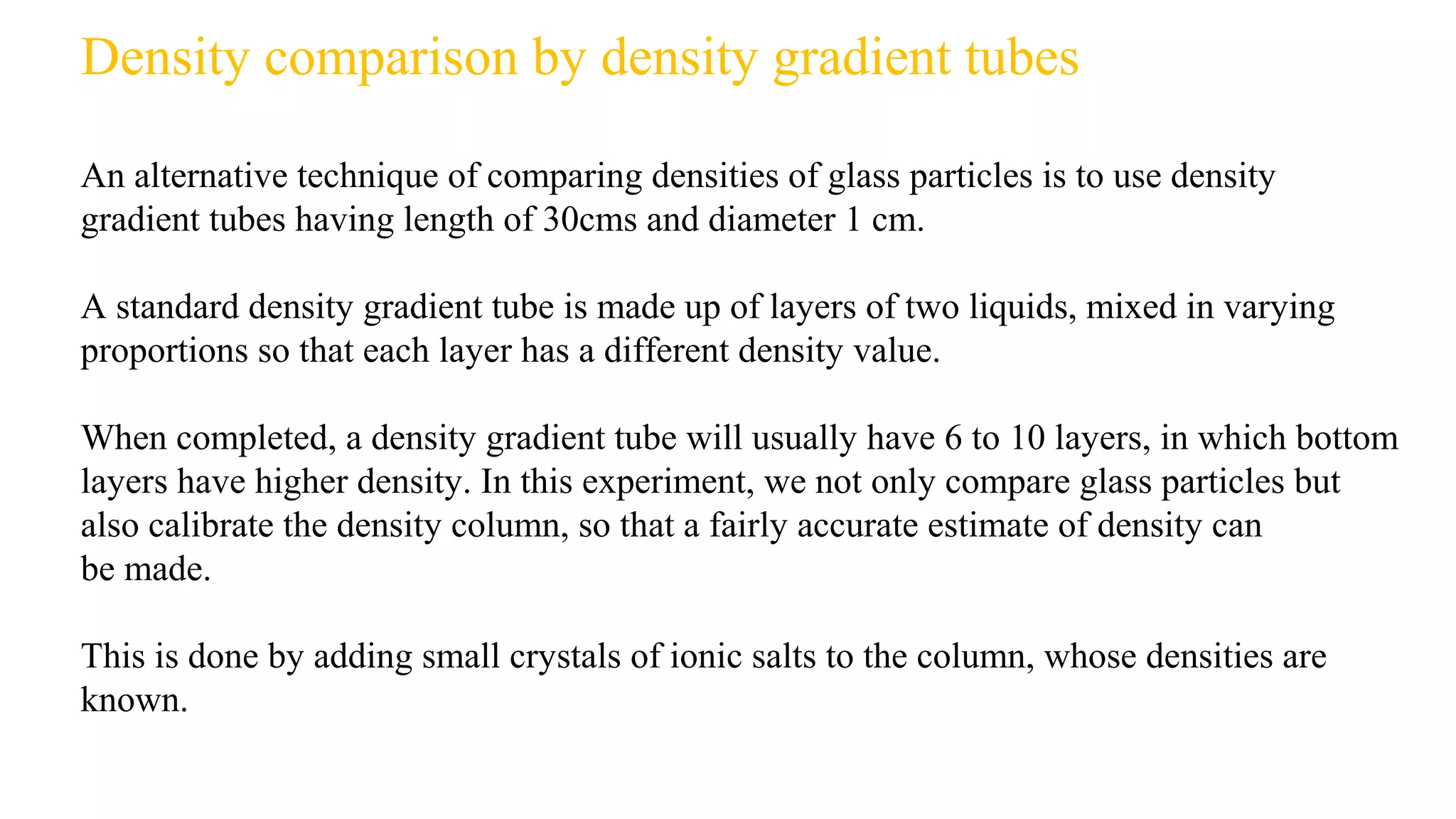
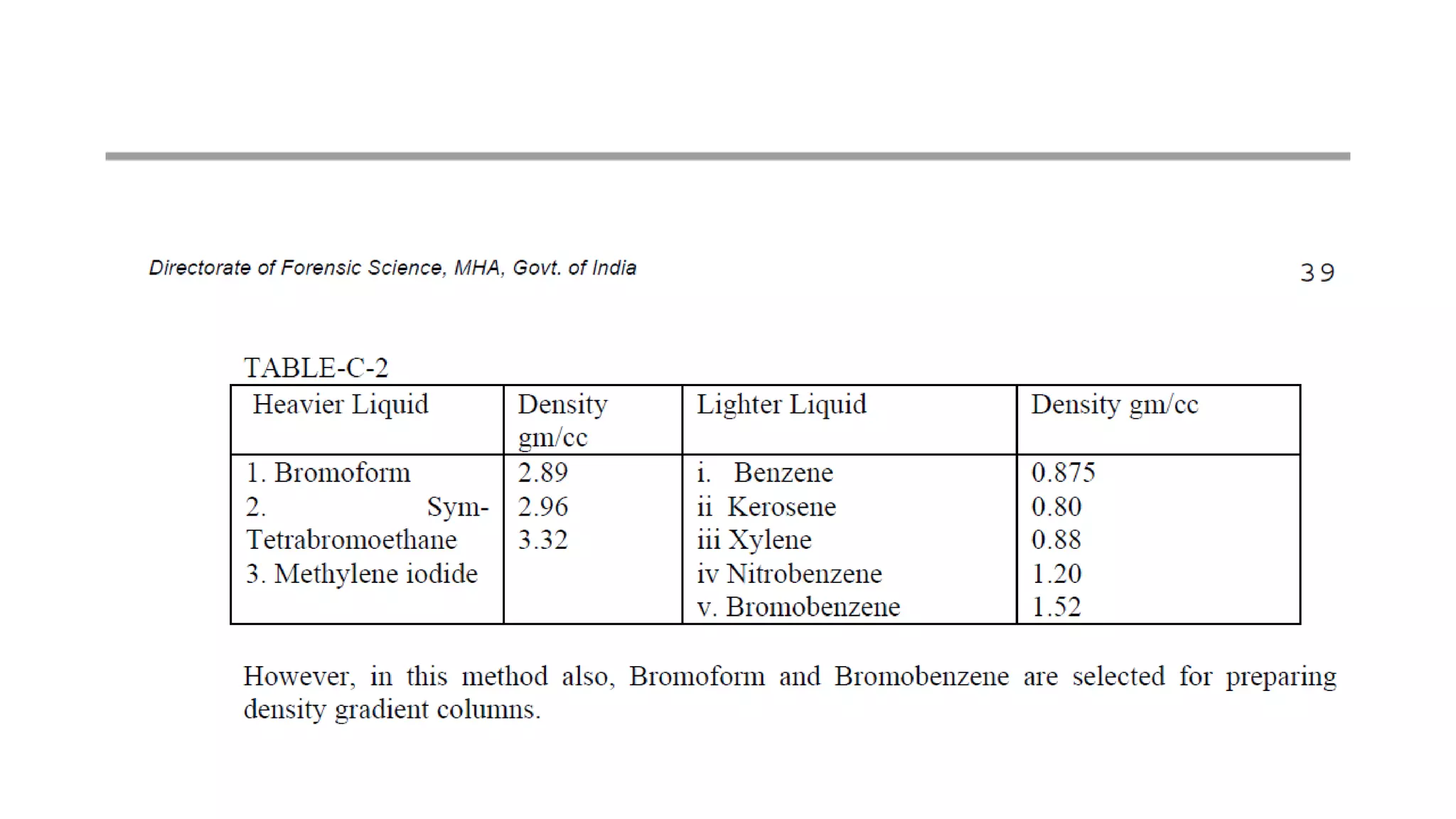
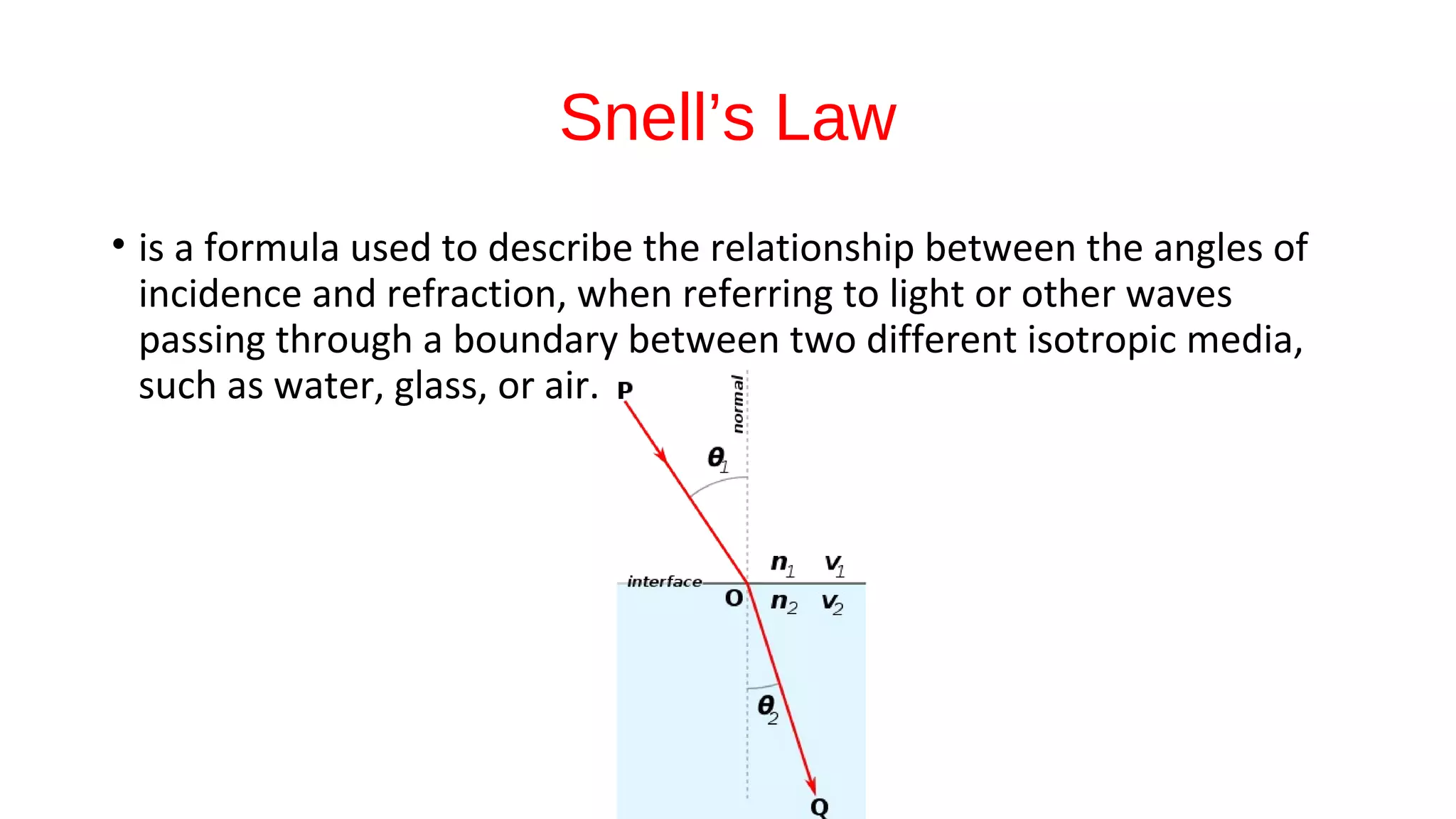
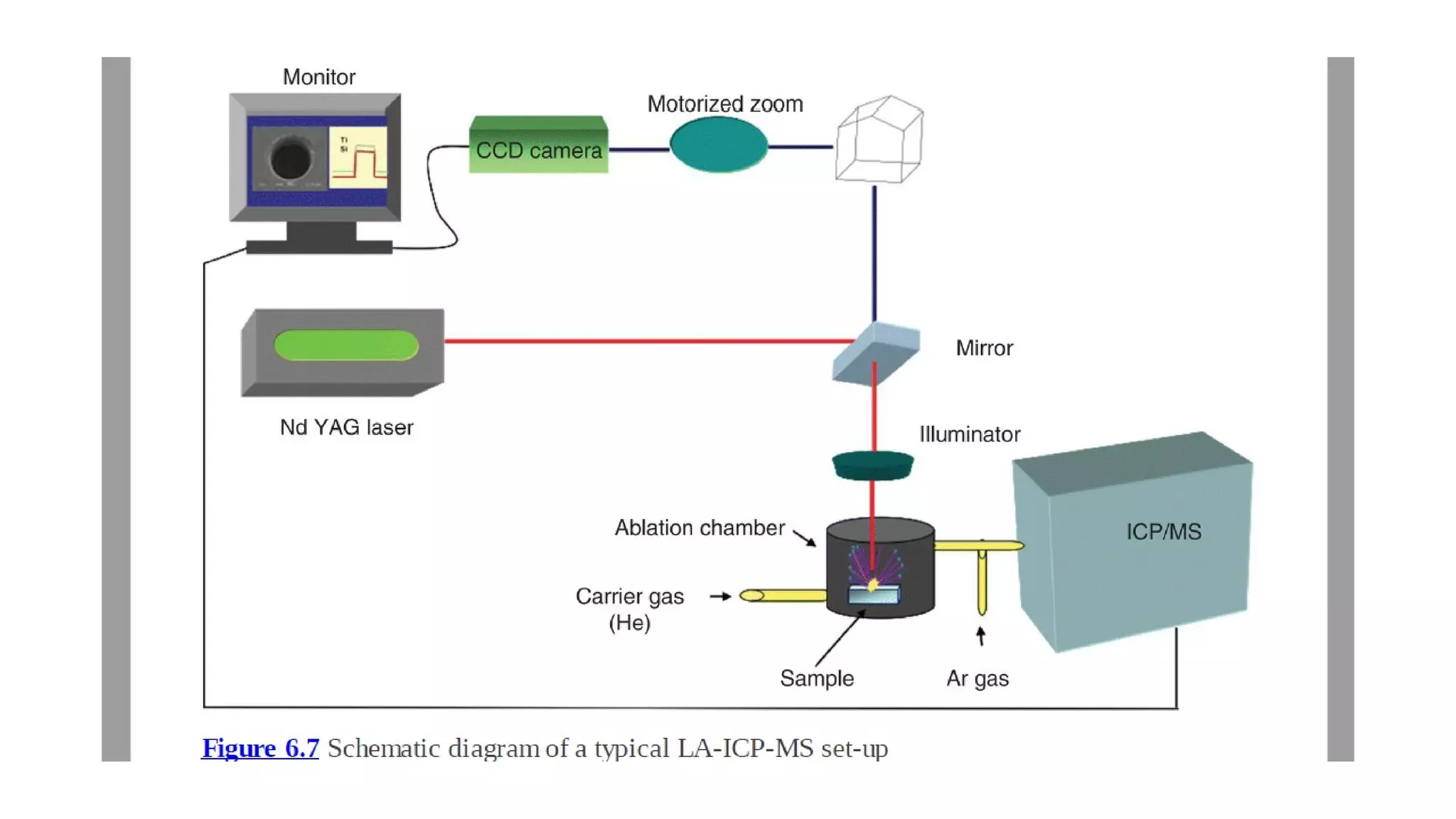
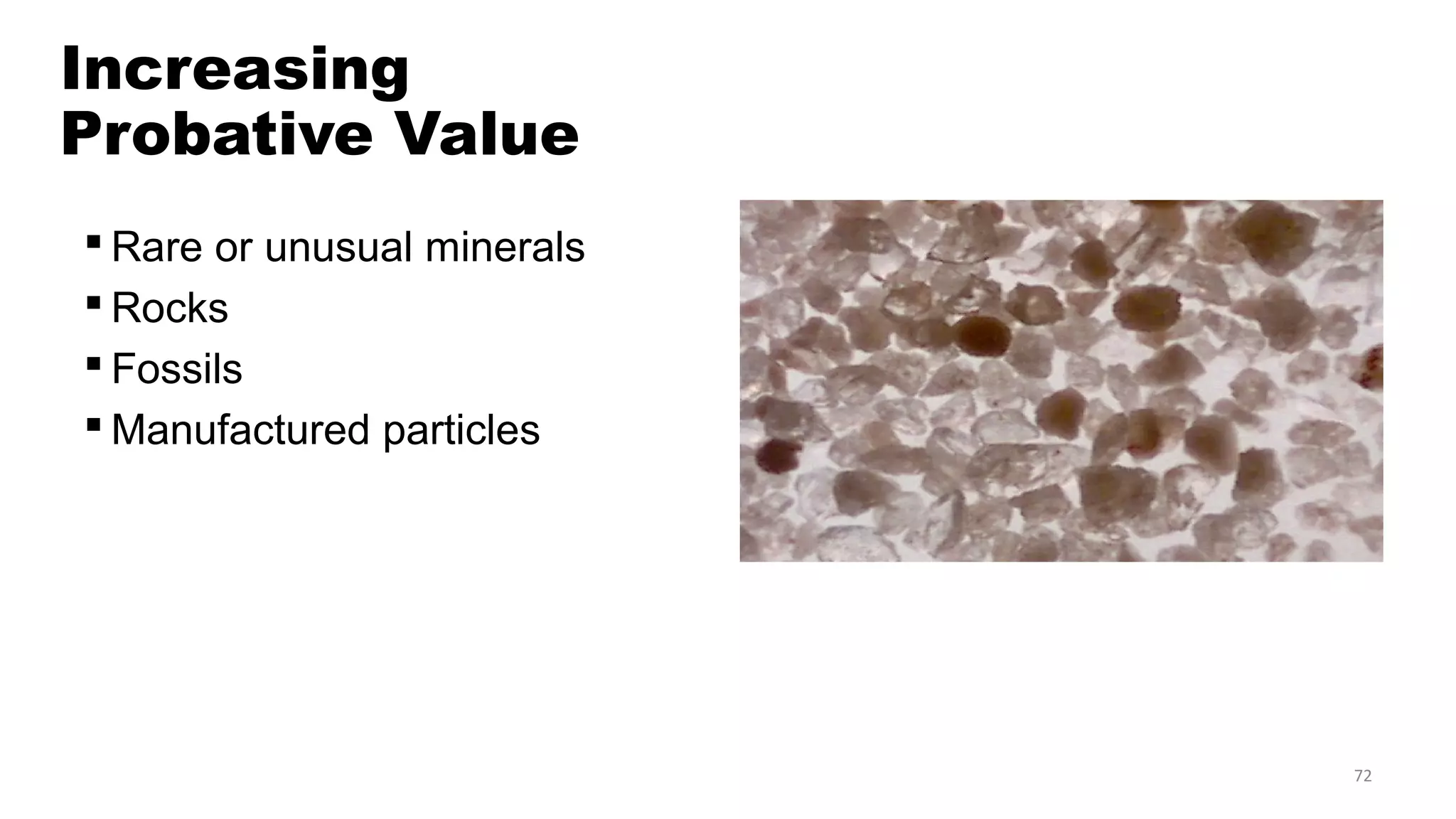
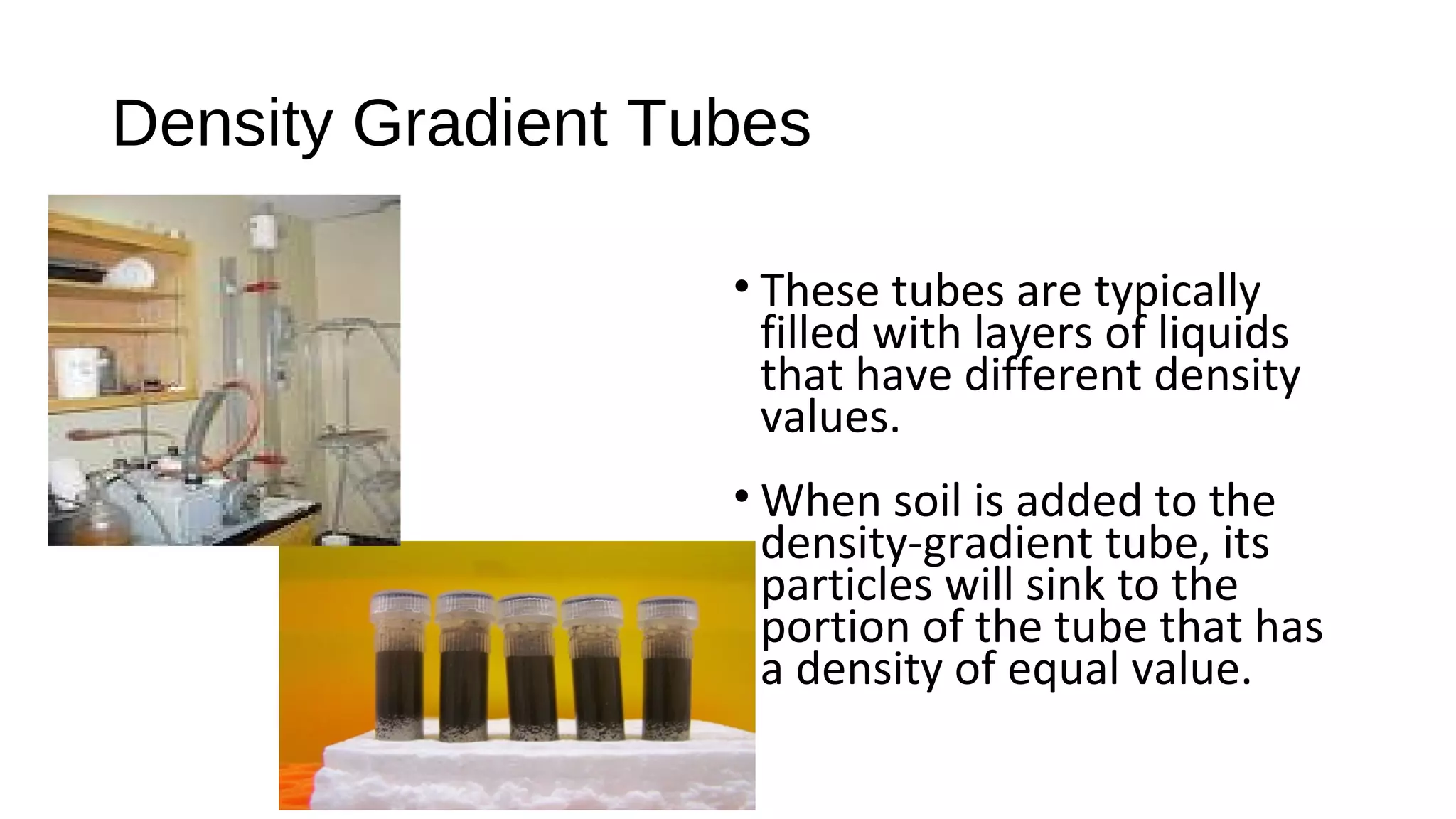
The document discusses the significance of glass evidence in forensic science, detailing its origin, physical and chemical properties, and how it can link suspects to crime scenes. Various types of glass are described, including their applications and manufacturing processes, along with methods for analyzing glass characteristics like thickness, curvature, and density. It highlights the role of glass as trace evidence and the meticulous examination required to establish links between glass fragments found at crime scenes.