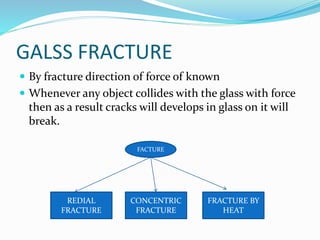
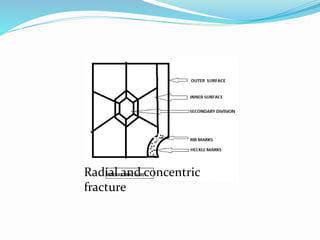
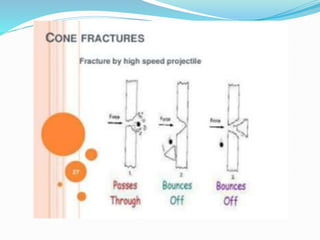
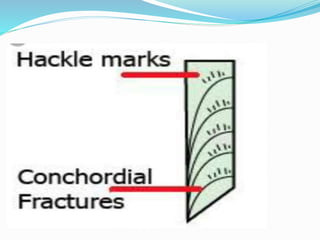
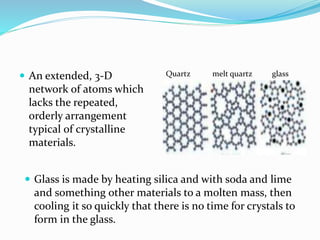
This document provides an overview of glass examination in forensic science. It defines glass and describes its amorphous internal structure. The document outlines the major types of glass based on manufacturing process and composition, and notes the most common uses. It discusses how glass fragments can be found at crime scenes and their evidentiary value. The document details how glass is collected and preserved as evidence. It explains methods for physical and chemical matching of glass, including examining refractive index, density, and fracture markings. It provides examples of common fracture patterns like radial and concentric fractures. In summary, the document serves as an introduction to the forensic analysis of glass evidence.











![TYPES OF GLASS
[A]. On the basis of manufacturing process:
Ordinary sheet glass
Float glass (plate)
[B].On the basis of composition:
Oxide glass
Non oxide glass
[C].On the basis of market application
Commercial the basic of market application :
Lead glass
Borosilicate glass
Laminated glass
Tempered glass](https://image.slidesharecdn.com/anjalipptglass-191115182303/85/Glass-fracture-13-320.jpg)