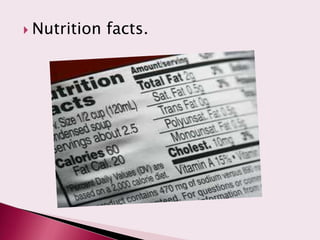
The document discusses food labelling requirements. It states that labels must be in English and include the product name, ingredients list, nutrition facts, quantity, expiration date, and manufacturer information. The most important rule is that labels cannot mislead consumers. Certain claims like unauthorized health claims are prohibited.