This document discusses food labeling requirements in the United States. It covers several key points:
- The principal goals of food labeling are to provide consumers with factual and relevant information about ingredients and nutrition. Required labels include the product name, net quantity, ingredients list, and responsible firm contact information. Voluntary labels can include health and nutrient claims.
- Labels must be legible and prominently displayed. Specific formatting and font size rules apply to various label elements like the nutrition facts panel and ingredient list.
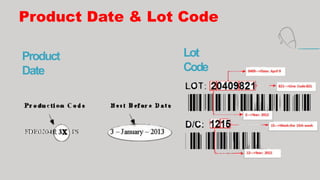
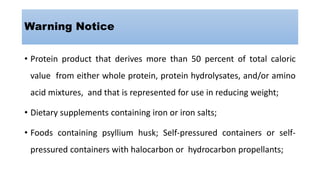
- Additional labeling is required for foods containing major food allergens, perishable items that need refrigeration, organic foods, raw meat/poultry (with safe handling instructions), and products that require