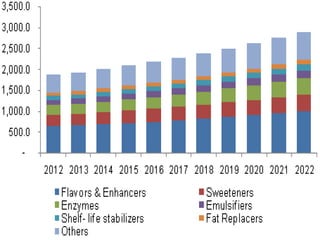
The document discusses food additives, which are substances added to food during processing, preservation, packaging, transportation or storage. Food additives can be natural or synthetic derivatives and are regulated to ensure safety. They have several functions like preserving food or improving taste and appearance. While approved additives are considered safe in limited amounts, consuming excess amounts can cause health issues like allergies or gastrointestinal problems. Stringent regulations evaluate additives for safety and require labeling with E-numbers in the EU. Both the EU and US have positive and negative lists to regulate approved and prohibited additives.