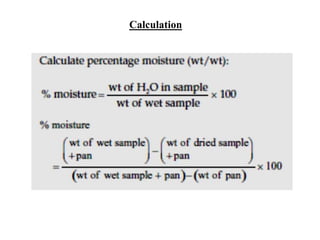
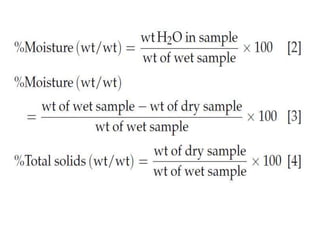
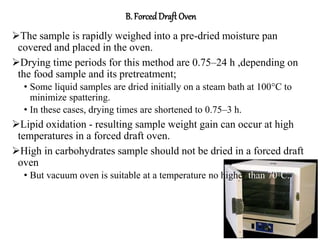
This document discusses the importance and methods of determining moisture content in foods. It notes that moisture determination is important for quality control, processing, labeling, and microbial stability. Common methods include oven drying, where the sample is heated and weight loss determines moisture content. Factors like temperature, time, sample size and type must be controlled to avoid decomposition. Moisture can exist in different forms within foods, from bulk water to chemically bonded water. The appropriate drying method depends on the food type and desired accuracy.


![Importance of determination of moisture content in food stuff
• Moisture content is one of the most commonly measured properties of food
materials. It is important to food scientists for a number of different reasons:
01] Legal and Labeling Requirements: There are legal limits to the maximum
or minimum amount of water that must be present in certain types of food.
02] Economic: The cost of many foods depends on the amount of water they
contain - water is an inexpensive ingredient, and manufacturers often try to
incorporate as much as possible in a food, without exceeding some maximum
legal requirement.
03] Microbial Stability: The propensity of microorganisms to grow in foods
depends on their water content. For this reason many foods are dried below
some critical moisture content.
04] Food Quality: The texture, taste, appearance and stability of foods depends
on the amount of water they contain.
05] Food Processing Operations: A knowledge of the moisture content is often
necessary to predict the behavior of foods during processing, e.g. mixing,
drying, flow through a pipe or packaging.](https://image.slidesharecdn.com/moisturedetermination-180417094902/85/Moisture-determination-and-Total-solid-3-320.jpg)